Fluorine Notes, 2009, 67, 1-2
ELECTROCTROCHEMICAL SYNTHESIS OF PERFLUOROPOLYETHERS USING KOLBE'S METHODN. V. Peganova, A. A. Ludikainen, V. A. Matalin, T. V. Mikchailova, N. V. Puzanova, V. V. Loginova, G.I. KaurovaRSC "Applied Chemistry", St.-Petersburg, Russia Abstract Here we have studied the process of electrochemical dimerization of dimer's and trimer's acids of hexafluoropropylene oxide (HFPO) at glass carbon SU-2000 and massive platinum in acetonitrile over water at variable concentrations of initial compound and different grade of neutralization of initial acid as well as the chance of obtaining some perfluoroethers within one process using cross condensation of several initial perfluorocarboxylic acids with further dividing of products by physical and chemical methods. The synthesis of perfluoroethers and partly fluorinated compounds using Kolbe method is of great interest, as the direct obtaining methods such as electrochemical fluorination lead to big losses of reagents due to destroying of carbon bonds with heteroatoms under harsh conditions [1]. Besides that, this type of electrosynthesis can result in combination of perfluorinated and hydrocarboxylic radicals. Synthesis mentioned doesn't require complicated apparatues and has as a rule high yield of dimerization reactions products. On the basis of obtained data and using the nanoelectrocatalysts it is possible to develop a method of carboxylic acids electrochemical oxidation, resulting in generation of alkyl radicals and in obtaining of precious (in practical terms) fluorine containing polyethers of common formula: High thermal stability (up to 350 oC) and chemical stability of these compounds defines their wide range of practical use for different fields of techniques. Their large temperature range of liquidity makes them irreplaceable for electronic industry as heat-transfers and solvents for production of printing circuit boards and other components of electronics. Polyfluorinated compounds may also be used as hydraulic fluids and lubricating oils [2] under extreme chemical and thermal influences. Kolbe synthesis is anodic dimerization of monocarboxylic acids or monosubstituted ethers of dicarboxylic acids at simultaneous decarboxylation of acidic residua: The direct Kolbe synthesis, when homogenious acids are interacting, as well as the cross one (when heterogenious acids are interacting, including acids with perfluorinated and/or hydrocarboxylic radical) is possible. In the last case the forming of several products can happen simultaneously, for example: Classic Kolbe electrosynthesis passes with participation of free radicals [3] generated near or on the surface of anode [4]. However, for a long time they couldn't manage to fix radicals at electric oxidation of perfluorocarboxylic acids, which was probably due to both very small period of radicals life and the possibility of their adsorption on the surface of electrode [6]. One of the main advantages of electrochemical dimerization is its high selectivity: due to adsorption at a certain material of anode only certain acidic groups are subject to decarboxylation under desired conditions, for which potential and other conditions of entering into near-electrode layer are optimal. The selectivity of Kolbe reaction is to a significant extent also defined by the properties of intermediate radicals, their life period, adsorbability, movability and others. If radicals formed on anode are inclined to further electrochemical oxidation into carbocation, then the characteristic products of Kolbe reaction will not be formed [6]. The particular feature of Kolbe reaction is its passing at high positive potentials. The material of anode influences greatly the direction of carboxylate electrochemical oxidation reaction [7], that?s why a number of authors [8, 9] have studied also the influence of electrode material nature on the process. The Methods of Experiment The dimerization was conducted in a glass non-diaphragm electrolyzer of 80 ml volume equipped with backflow condenser and bubbler as a mixing device. A plate made of SU-2000 glass carbon or heavy platinum was used as an anode. The surface of anode was 16 cm2 in all cases. Stainless steel was used as a cathode. The experiments were conducted in the mode of periodical charging of electrolyte, the ending of reaction was defined by the growth of tension at resinification of electrolyte or by the stop in product formation. The concentration of main compound in the initial acids of dimer and trimer of hexafluoropropylene oxide was 90% and excluding the experiments mentioned separately 0,05 - 0,5 of NaOH were neutralized. Oligomers of hexafluoropropylene oxide and their acids are similar in physicochemical properties, therefore dimer usually contains up to 10% of trimer and traces of tetramer , trimer contain the impurities of dimer and tetramer. Initial acids, crude products, fractions after neutralizations were analyzed chromatographically. Electrolyte composition: 35% mass. acids-reagents (partly neutralized with soduim alkali to provide conductivity for electrolyte solution), 50% of acetonitrile, 14% of water. Current was 1,6 A , voltage 5-9V. The isolation of products of electrosynthesis was done at a column of 25cm long packing nozzle helical nozzle. Results and Discussion The conditions and results of perfluorinated acids dimerization reactions are listed in tables and illustrated. It is known from literature [8, 9, 10] and based on the experience of our work, that the best results at using the trimer acid of hexafluoropropylene oxide have been reached at its initial concentration of HFPO 35%. We could not find the analogous data for dimer acid of hexafluoropropylene oxide to use for planned solutions, that is why in experimental way we had chosen the optimal conditions for conducting of electrolysis, they provided the longest period for that as well as the biggest yield of targeted product in terms of current and compound. Table 1. The dependency of PE-125 (perfluorine-5,6-dimethyl-4,7-dioxadecane) polyether dimerization reaction products yield and process duration on the concentration of initial dimer acid of hexafluoropropylene oxide. Electrode material SU-2000, current density 0,1 A/cm2, U = 6 -9,5V, solvent: CH3CN acetonitrile and 14% of water, at the neutralizing using NaOH alkali of ten mole percents of acid
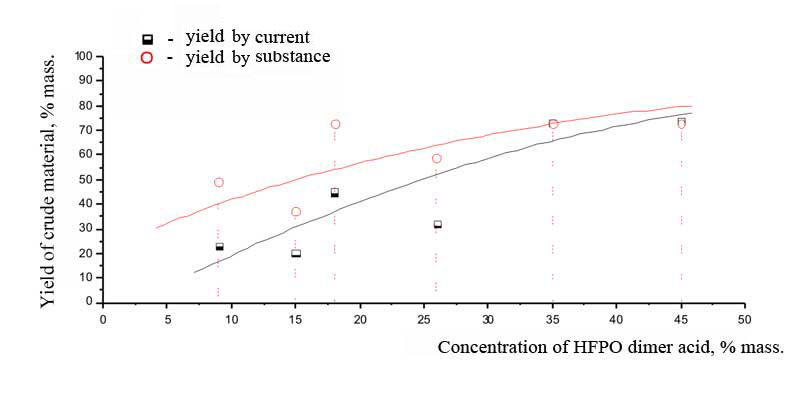
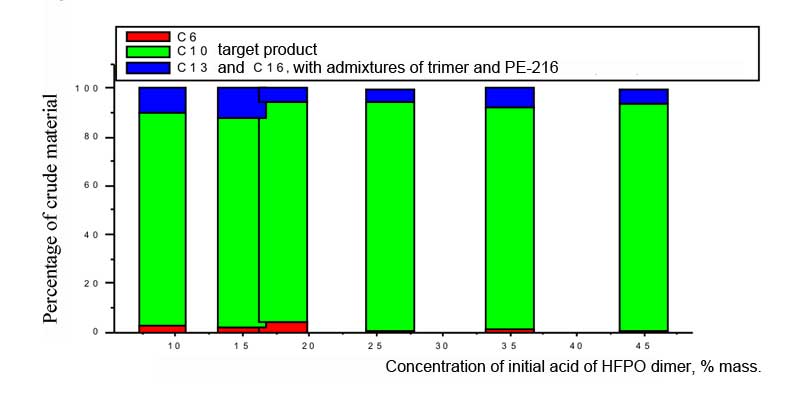
The electrolysis at the lowest concentration of dimer acid of hexafluoropropylene oxide has been stopped because of the strong growth of voltage at constant load and because of significant resinification of eletrolyte. In all other experiments the voltage dropped with the lapse of time from 8-9 V to approximately 6 V. According to Table 1 and Picture 1 it is seen, that at small concentrations of dimer acid of HFPO the mistake of measuring of results obtained was great. When the concentration of initial acid is about 35% and more the yields of crude product by current and by substance virtually coincide and reach maximum , but according to Picture 2 we can see, that the content of target polyether PE-125 at 45% concentration of initial dimer acid of HFPO has decreased, therefore the optimal concentration of reagent under that conditions at SU-2000 must be about 35% mass, as for the earlier [8, 11] studied trimer acid of hexafluoropropylene oxide. According to the chromatographic analysis of reaction products we can also see, that at optimal concentration of the initial acid the share of by-product - perfluorine-5,8,9,12-tetramethyl-4,7,10,13-tetraoxahexyldecane (PE-216) is bigger, which is easily separated from the target product. Pic. 1. The dependancy of crude product yeild on the concentration of dimer acid of hexafluoropropylene oxide. Electrode material SU-2000, current density 0,1 A/cm2, U = 6 -9,5 V, solvent: acetonitrile CH3CN and13% of water, neutralizing using NaOH alkali of ten mole percents of acid. Besides that, according to the results of chromatographic analysis (Pic. 2) it can be seen, that at lowest concentrations of initial dimer acid of hexafluoropropylene oxide a small amount of initial products remains in crude product, that amount virtually dissapears by incerasing the concentration of reagent. It could be because of the fact, that at decreasing the amount of initial acid the amount of reactions of radicals with solvent and resinification is increasing, as a result of which we have growth of voltage in electrolyzer, changing of conditions of adsorption of electro-active particles and other undesirable changes up to destruction of reagents and stopping of electrode reaction. Pic. 2. The dependancy of crude product composition on the concentration of initial dimer acid of hexafluoropropyleneoxide. Material of electrode SU-2000, current density 0,1 A/m2, U = 6 - 9,5 V, solvent: CH3CN acetonitrile and 14% of water, neutralization using NaOH alkali of ten mole percent of acid Table 2. The dependancy of polyethers yield and duration of electrolysis process on the percentage of neutralization of initial dimer acid of hexafluoropropylene oxide. Electrode material SU-2000, current density 0,1 A/m2, U = 6 - 9,5 V, solvent: CH3CN acetonitrile and 14% of water
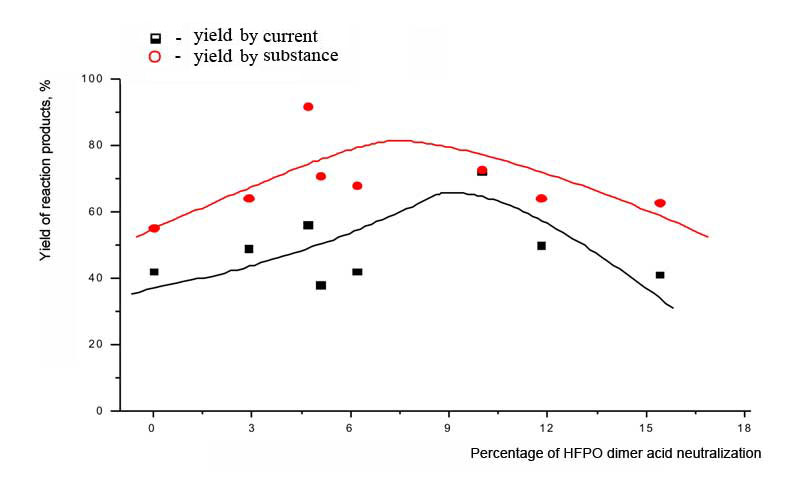
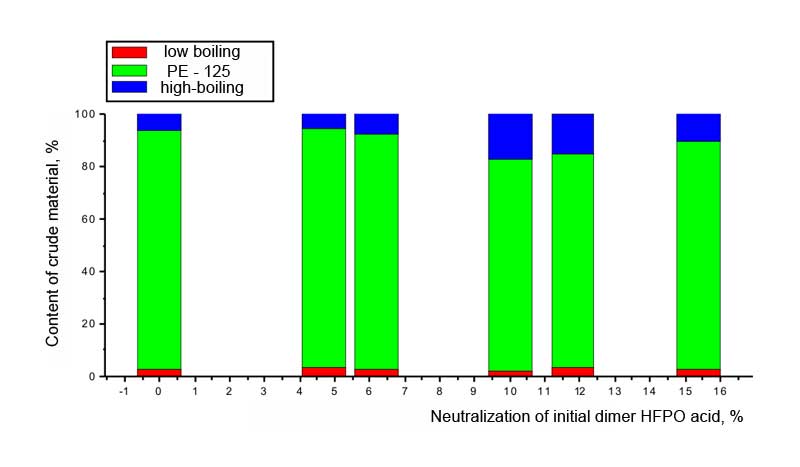
We also have studied he depedance of parametres of perfluoropolyether synthesis out of dimer acid of hexafluoropropylene oxide on the percentage of neutralization of the initial reagent. At first, this provides the constancy of concentration of background electrolyte; secondly, the acidity of initial reagent lowers and the probability of radical mechanism of reaction increases - without transfer of formed radical into carbcation; and, at last, thirdly, as a result of adding the alkali a buffer solution is formed of a certain capacity, which provides the constancy of conditions of adsorption of reaction participants at anode. It has been stated, that as opposed to trimer acid of hexafluoropropylene oxide, the dimerization of which passes at optimal neutralization of 5% (mole) of initial acid the dimerization of dimer acid of hexafluoropropylene oxide requires higher percentage of neutralization: 8-10 mole %. This can be due to the bigger strength of dimer acid of hexafluoropropylene oxide. Pic. 3. The dependancy of perfluoropolyether raw product yield at current and compound on the percentage of neutralization of the intial dimer acid of hexafluoropropylene oxide However, according to the Pic. 4 we can see, that at increasing of alkali concentration in the initial electrolyte the share of high-boiling admixtures in crude product rises and the amount of target product decreases. Pic. 4. The dependancy of raw product composition on the share of neutralization of initial dimer acid of hexafluoropropylene oxide according to the data of GLC analysis. Thus, the increasing of side reactions amount doesnt allow the increasing of percentage of neutralization of dimer acid of hexafluoropropylene oxide, in spite of the significant prolonging of the process. As modern conceptions of perfluoropolyethers electrosynthesis by the mentioned method include the stage of preliminary adsorption of reagents [9, 12], the change of anode material's nature should influence significantly the process of perfluoroacids dimerization. Therefore besides the experiments at SU-2000 the synthesises at massive platinum as an anode were also conducted. Taking into account the previous series of experiments we have chosen the optimal concentration of dimer acid of hexafluoropropylene oxide with minimum and maximum grade of neutralization using sodium alkali. The results are listed in Table 3. Table 3. The dependancy of perfluoropolyether-125 (PE-125) crude product yield at platinum anode on the percentage of neutralization of the initial dimer acid of hexafluoropropylene oxide. Electrode material is massive complanate platinum, current density 0,1 A/m2, U = 5,2 -8,1 V, solvent: CH3CN acetonitrile and 14% of water, neutralization by NaOH alakli of part mole percents of acid.
Comparing to the data listed in Table 2 it can be seen, that the process duration had increased almost twice and the amount of obtained raw product had more than tripled. The yields at current and at compound had increased respectively. The experiments with dimerization of trimer acid of hexafluoropropylene oxide were also conducted at SU-2000 anodes and platinum anode. As opposed to the initial dimer acid of hexafluoropropylene oxide bigger yields of crude product by current and by compound were obtained at glass carbon anode for trimer acid, however according to chromotographic data the percentage of target perfluoropolyether is bigger in crude product obtained using platinum anode. Table 4. The dependancy of perfluoropolyether-216 raw product yield at glass carbon anode and platinum anode on material of electrode. Current density 0,1A/m2, U = 4,2 - 9,1 V, solvent: CH3CN acetonitrile and 14% of water, neutralization by NaOH alkali of five mole percents of acid.
*Crude product yield by substance is 100% due to the fact, that the part of reaction products is a result of interaction between reagent and solvent. The content of pure target PE-216 is 89% in crude product according to data of GLC. The recounting onto target product of yield by current produces 87% and at platinum anode - less than 72%. However, yield by current even at recounting onto pure target product produces almost quantitve result according to chromotographic data. When washing off the crude product from electrolyte some differences in properties of PE-216 and PE-125 had been noticed. PE-216 is easily washed off with water, the emulsion sets fast and fractions easily separate at separating funnel. PE-125 is not being subject to demulsification from water so fast. Its emulsion separates much better with soda solution and not with the water. Additional peaks appear at chromotograms of PE-125 over water in samples (without additional drying). Besides that, we have studied the opportunity of joint dimerization and some other acids: trimer of hexafluoropropylene oxide, perfluorobutyric, perfluoroenantic, dimer of hexafluoropropylene oxide, perfluoropelargonic and valerianic, - including Kolbe cross electrosynthesises at SU-2000 and at massive complanate platinum. It is proved, that dimerization of perfluorobutyric acid doesn't occur at glass carbon, which is due probably to very small period of life of active radical formed at anode. It is confirmed as well by the fact, that in cross synthesises with other acids at glass carbon the yield of blended products was rather significant, besides that perfluorohexane was isolated. The reaction of cross synthesis of perfluorobutyric and valerianic acid at SU-2000 doesn't occur, the second acid has got a short carbon skeleton its radical's life period is not big as well. For some of the above mentioned reactions of cross synthesis we have proved an influence of electrolyte makeup as well as of nature of indifferent cation. Testing of the influence of cation's nature and concentration on the process of cross dimerization was conducted for dimer and tirmer acids of hexafluoropropylene oxide and perfluorobutyric acid. It was proved, that at neutralization of five mole percent of initial acids compare to ten percent the yield by current increased almost by ten percent and the yield by substance grew slightly. At the same time the replacement of sodium alkali for aqueous ammonia virtually doesn't influence main parameters of the process. As it has been noticed, perfluorobutyric acid doesn't dimerize at SU-2000, although in cross synthesises it may be a big part in the composition of the reagents. In accordance with the conception on the mechanism of Kolbe synthesis, when molecules of carboxylic acids form radicals at electrode and then move themselves away into the solution volume to react, the radicals of perfluorobutyric acid are either very short-living and therefore can't manage to react, or firmly adsorb at electrode and can react only with movable radicals of other acids or solvent. The last fact is proved by a big number of cross synthesises, in which perfluorobutyric acid radical of good yield takes part [9]. The result of cross synthesis of pefrfluorobutyric acid and dimer acid of hexafluoropropylene oxide has good prospects, the product of their interaction virtually conforms by its characteristics to commercially used perfluorodibutyl ether (DBE). Table 5. Comparing of DBE Characteristcs And Its Analogue C3F7-O-CF(CF3)-C3F7 (purity 97-98 % ): Physical, Chemical and Dialectical Properties
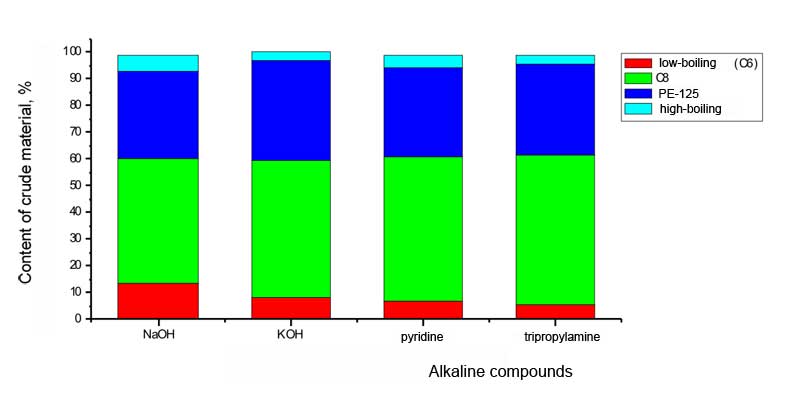
The influence of nature of neutralizing component of electrolyte on the yield has been proved for that process. Sodium and potassium alkali, pyridine and tripropylamine have been tested as well. It was found, that yields of crude material by current and by compound in the error limit do not depend on the nature of alkali, but according to the data of GLC the yield of C8 ether (perfluoropropyl-2-amyl or ME-99) are somewhat higher when using pyridine and tripropyamine, than over sodium and potassium alkali, and the yield of PE-125 polyether on the contrary is somewhat lower (refer to Pic. 5). Pic. 5. The Dependance of raw material composition on the nature of neutralizing component at electrochemical dimerization of mixture of dimer acids of hexafluoropropylene oxide and perfluorobutyric acid. Overall concentration of acids - 35%, ratio of mole concentrations 1:2. Anode of SU-2000, current density 0,01 A/m Cross dimerization of trimer acids of hexafluoropropylene oxide and perfluorobutyric acid was conducted at glass carbon anode of SU-2000 at variable percent of neutralization of initial acids. It was proved, that percent of neutralization doesn't influence the process, however, according to the data of GLC the content of main product of C11 (perfluoro-4,7-dimethyl-5,8,-dioxaundecane) in crude product goes up by 8-10% at increasing the percent of neutralization of the initial acids from 2-5 to ten mole percents. Nevertheless, the increasing of high-boiling products' share with the decreasing of alkali amount can also be of interest, as the larger part of this fraction is C13 polyether (perfluoro-5,6,9-trimethyl-4,7,10-trioxatridecane) - the product of cross synthesis of dimer and trimer of hexafluoropropylene oxide. When replacing the glass carbon anode for the platinum one the yield of crude product by current and by compound increases by approximately 10-12%, and the composition data doesn't practically change according to the choromatography. C11 polyether isolated out of electrolysis products' mixture of 96% mass. purity, possesses physical and chemical properties, listed in Table 6. Table 6. C11 perfluoropolyether : C3F7-O-CF(CF3)-CF2-O-CF(CF3)-C3F7 (96% purity) - its physical and chemical properties and dielectric characteristics
Conclusions 1. The processes of perfluoropolyethers' synthesis by dimerization of dimers and trimers of acids were investigated. The dependency of products' yields on the concentration of initial reagents and their neutralization grade had been proved at anodes made from SU2000 and massive platinum. The optimal parameters of the dimerization reaction had been selected for the most long period and productivity of the process. 2. The dependences of Kolbe electrosynthesis of trimer acids of hexafluoropropyleneoxide, perfluorobutyric, perfluoroenantic acids, dimer acid of hexafluoropropyleneoxide, perfluoropelargonic and valerianic acids on parameters of reactions had been studied. Significant increasing of yields of reaction products and duration of electrolysis at trasition from anode made of SU-2000 to electrode made of massive even platinum was proved. 3. The possibility of C8 and C11 perfluoroethers synthesis by cross electrodimerization of different perfluoroacids was proved. The processes pass with good yield at current, are stable in time and technological. Summary yield of useful products in electrosynthesises climbs over 90%. References 1. Knunyants, I.L., Polishchuk, V.R. Novye dannye o reaktsiyah ftororganicheskih soedinenij / I.L. Knunyants, V.R. Polishchuk // Uspekhi khimii. – 1976. – T. 45, v. 7. – S. 1139–1176 2. Tarrant P., Stamp E. Okisi perftorolefinov // ZhVKhO im. D.I. Mendeleeva. – 1970. – T. 15, № 1. – S. 34-44 3. A.K.Vijh, B.E. Conway Electrode kinetic aspects of the Kolbe reaction // Chem. Rev. – 1967. – V. 67. – P. 623–627 4. Grinberg, V. A. Anodnye protsessy s generatsiej radikalov i ion-radikalov v sinteze ftororganicheskih soedinenij: avtoref. dis doktora khim. nauk : 02.00.08 / Grinberg Vitalij Arkad'evich. – Moskva, 1992. – 43 s. 5. Tumanskij, B.L. Elektrokhimicheskij sintez perftoralkilhloridov / B.L. Tumanskij, V.A. Grinberg, Yu.B. Vasil'ev, N.N. Bubnov, L.L. Gervits, K.N. Makarov // Elektrokhimiya. – 1990. – T. 26, v. 8. – S. 1052 6. Knunyants, I.L. Uspekhi sinteza i issledovaniya ftororganicheskih soedinenij/ I.L. Knunyants, V.R. Polishchuk // Uspekhi khimii. – 1975. – T. 44, vyp. 4. – S. 685–714 7. Nadelbaum P.R., Fahidy T.Z. The oxygen film on platinum electrodes and the electro oxidation of acetate ions // Electrochim. Acta. – 1972. – V. 17, №9. – P.1659 8. Chechina, O.N. Elektrohimicheskie sintezy na osnove ftorkarbonovyh kislot : diss. doktora khim. nauk : 02.00.08 / Chechina Ol'ga Nikolaevna. – Shymkent, 1993. – T.1, 374 s. 9. Cherstkov, V.F. Zavisimost' lokalizatsii elektrohimicheski generiruemyh radikalov ot ih stroeniya i prirody rastvoritelya/V. F. Cherstkov, V. A. Grinberg, S. R. Sterlin, Yu.B. Vasil'ev, L.S. German, E.I. Mysov // Izvestiya AN SSSR, Seriya khimicheskaya. – 1991, № 5. – S. 1134-1141 10. Patent SU 546167 . Sposob polucheniya polioksaperftoruglerodov / Berenblit V.V., Panitkova E.S., Trusova E.Ya., Sokolov S.V., Publ. 15.10.1976, Bull. № 53. – 6 s. 11. Razrabotka elektrohimicheskogo sposoba polucheniya oktaftorbutana (hladona 338) : otchet o NIR / MGO Tekhnokhim, NPO GIPKh; ruk. I.L. Serushkin. – SPb., 1992. – 17 s. – Inv. № 110-92 12. Grinberg, V.A. Elektrokhimicheskaya generatsiya i povedenie stabil'nyh perftorirovannyh radikalov na platine i steklouglerode /V.A. Grinberg, B.L. Tumanskij, N.A. Majorova, Yu.B. Vasil'ev, L.L. Gervits, K.N. Makarov, E.A. Avetisyan, V.F. Cherstkov, S.R. Sterlin, L.S. German// Elektrokhimiya. – 1991. – T.27, v. 10. – S. 1374-1377 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fluorine Notes, 2009, 67, 1-2