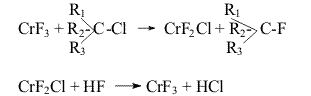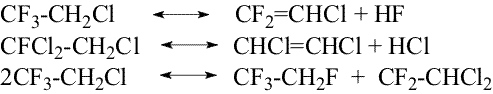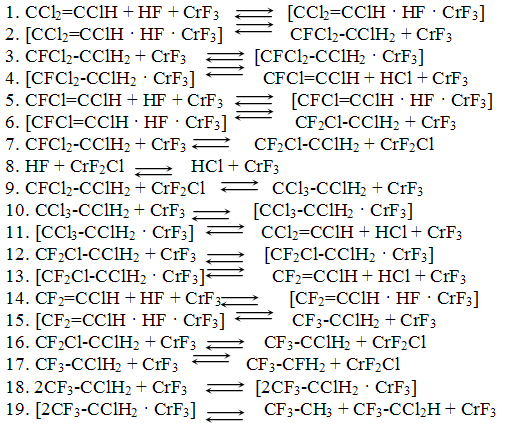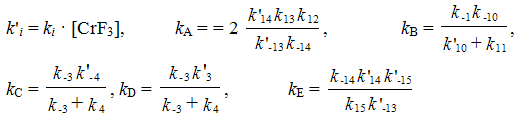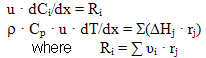Fluorine Notes, 2009, 66, 1-2
TECHNOLOGIES FOR PRODUCTION OF CHLADONS BY A METHOD OF GAS PHASE CATALYTIC HYDROFLUORINATION Federal State Unitary Enterprise "Russian Scientific Center
"Applied chemistry" Chlorofluorocarbons and bromofluorocarbons (chladons and halons) as compounds possessing a number of unique properties such as chemical inertness, non-toxicity, explosion and fire safety, were widely used in engineering as aerosol propellants, refrigerants, foaming agents for plastics, solvents, high-performance extinguishers etc. But the ozone depleting property of some chlorine- and bromine-containing compounds revealed in the 80-ties attracted attention to a large group of industrial chladones as potentially dangerous substances containing chlorine and bromine in their composition. The Montreal Protocol (MP) on substances that deplete the ozone layer was adopted in 1987y. As by this moment the depleting role of chlorine and bromine had been confirmed by a number of research teams, the Addendum to the MP listed ozone depleting substances (ODS) subject to regulation by all countries being the parties to the MP. This Addendum contained chlorofluorocarbons (CFC), bromofluorocarbons (halons) and some chlorohydrocarbons (CHC). The MP obliged the Parties to reduce consumption, production and import/export of ODS. The Soviet Union was one of the first-rate manufacturers of ODS: in 1987y its share in ODS production made 15% of the world volume (approximately 200 thousand tons). At the same time the Soviet Union was one of the largest consumers and outstanding exporter of ODS that supplied not only all republics of the former USSR but also a whole number of European and Asian countries. Six large chemical plants in Russian Federation produced ODS. Almost all industry branches in RF were consumers of chladones [1,2]. FSUE RSC "Applied Chemistry" together with a number of research institutes of RF have made a great effort to convert domestic industry to a new class of chemical substances instead of the prohibited ODS. As a result of their investigations a nomenclature of new chladones was suggested: hydrofluorocarbons 134a (CF2CFH2), 152a (CF2HCH3), 125 (CF3CF2H), 32 (CH2F2) etc. The main difference of ozone-friendly compounds from ODS is that their molecules does not contain chlorine and bromine atoms that may participate in "bromine" or "chlorine" cycle of the ozone depletion. Besides the zero value of ozone depleting potential (ODP) and the value of global warming potential (GWP), the main criteria in the choice of ODS alternatives is proximity of physical, chemical and maintenance properties to the analogues characteristics of ODS to be replaced. The main nomenclature of ozone friendly compounds adopted for development and
implementation into the industrial manufacture in Russian Federation is given
in Table 1.
Table 1. Continuation
But the liquid phase hydrofluorination method has a number of limitations: Taken that into account to obtain hydrofluorocarbons with a great fluorine content in the molecule (CF3-CFH2, CF3-CF2H) the gas phase catalytic method of hydrofluorination is used. The peculiarities of gas phase catalytic hydrofluorination are seen on the example of 1,1,1,2-tetrafluoroethane ( HFC-134a) synthesis from trichloroethylene and hydrogen fluoride. The synthesis process runs in two stages (Scheme 1): It follows from the experimental data and thermodynamic calculation of stage (1) of the process that HFC133a content in the organic share of the synthesis products may reach 90-98%. Stage (2) is reversible. In dependence on the conditions the contentof HFC 134a in the organic share of the synthesis products may make 10-40% by volume (from organic part of the synthesis products). For the process there is used a chromine-magenesium-fluoride catalyst [3, 16] manufactured from magnesium fluoride powder by saturation with chromium chloride solution followed by mixing, moulding, drying and treatment with hydrogen fluoride with the purpose to convert chromium chloride (CrCl3) into chromium fluoride (CrF3) [4, 5]. The study of the mechanism of the catalyst effect in the present process has shown that the catalyst acts as a carrier of fluorine and chlorine atoms [6-8]. It is suggested that the process of hydrofluorination of chlorofluoroethanes runs according to scheme (2):
where chromium fluoride substitutes chlorine by fluorine in chlorofluoroethanes changing its composition to CrF2Cl,after that hydrogen fluoride replaces chlorine with fluorine in active catalyst part to form hydrogen chloride and CrF3. This mechanism seems more probable than mechanism [9, 10] that contains the stage of interaction of HF and chloroalkane molecules absorbed on neighboring active centers of the catalyst. Besides the main hydrofluorination processes this catalyst is used for the reactions of dehydrofluorination, dehydrochlorination and disproportionation (Scheme 3) The obtaining unsaturated fluorine-containing compounds( particularly CF2=CHCl
that forms azeotrope with 1,1,1,2-tetrafluoroethane) in future make more
difficult the desired product separation from the reaction mixture. Obviously
the mechanism of these processes is connected with formation of active transition
complexes on the catalyst surface.
On the basis of the suggested mechanism with the use of the method of routes for complex reactions 11 a kinetic model for the process of synthesis of 1,1,1,2-tetrafluoroethane has been developed [12]. 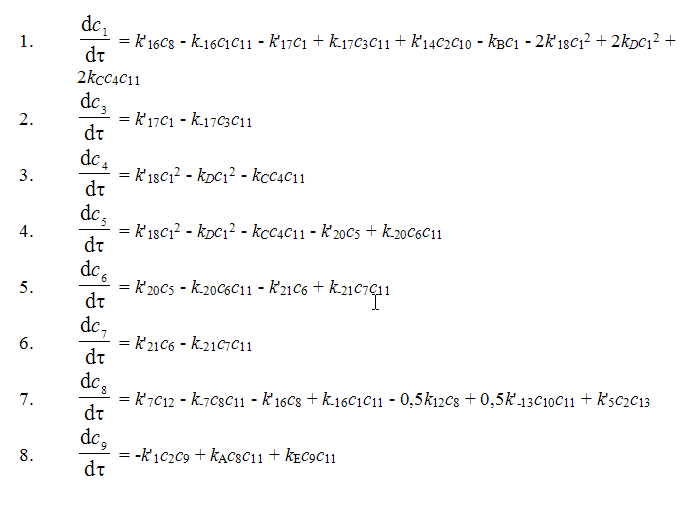 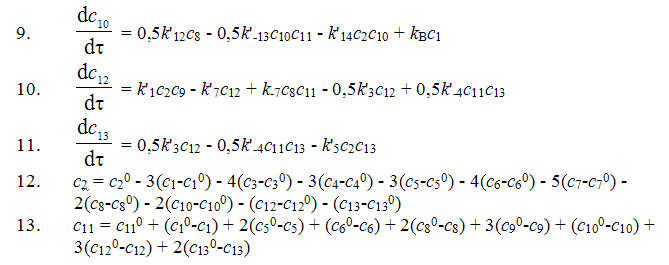
where: c1 is concentration of CF3-CClH2, In the search of the kinetic model constants for numerical integration of the
system of ordinary differential equations there was used LSODA method [12].
The found values of the kinetic constants are given in Table 2.
The process analysis with the use of the kinetic model has shown that the optimal conditions for stages (1) and (2) (Scheme 1) substantially differ (exothermic and endothermic processes), stage (1) is to be carried out at a lower temperature in comparison with stage (2) etc. Therefore the synthesis of HFC 134a is reasonable to carry out according to the two-reactor scheme. For the choice of the optimal scheme of the reactor unit there was used a mathematical model of adiabatic reactor with stationary bed of chromium-magnesium-fluoride catalyst: Ci = concentration of component i in the gas mixture, mole/m3; u = linear velocity
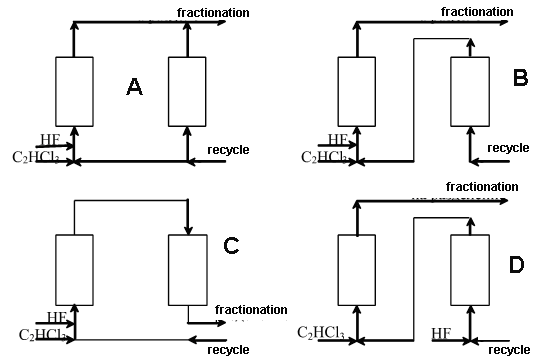
of the mixture, m/s; Fig.1. Variants of two-reactor scheme of 1,1,1,2-tetrafluoroethane synthesis The calculations according to the above stated mathematical model have shown
that the maximal output of HFC-134a provides variant A [13] ( see Table 3).
According to variant A the first reactor is fed with hydrogen fluoride, trichloroethylene and part of the recycling mixture( to hold the temperature regime) in which the total content of HF and 1,1,1-trifluorochloroethane is more than 99%, the second reactor is fed with main part of the recycling mixture ("recycle") of the same composition; the flows from the reaction zones are united and directed to separation of hydrogen chloride, low-boiling products and 1,1,1,2-tetrafluoroethane while the high-boiling substances are returned as a recycle to the synthesis unit. The standard process flowsheet for synthesis of ozone friendly HFCs has been developed by the method of gas phase catalytic hydrofluorination ( pentafluoroethane, for example) [14]. The technology of production of HFC116 [15] was developed for processes of plasmachemical etching in microelectronics and the technology for HFC 23 [17] manufacture was developed for filling low-temperature refrigerating machines. Industrial facilities for ozone friendly HFCs in Russian Federation have been
created and are creating on the basis of the investigations carried-out.
2. V.G. Barabanov, O.V. Blinova, V.S. Zotikov, S.A. Lizgunov, A.P. Orlov, G.D. Orlov, V.B. Rusanov, V.I. Samojlenko, I.G. Trukshin, V.N. Tselikov, Ozolnobezopasnye al'ternativy i zameniteli. Propellenty, hladagenty, vspenivateli, rastvoriteli, ognegasyashchie sredstva, Khimizdat, Sankt-Peterburg.-Moskva, 2003, 304 s. 3. Patent RU 2051139, 1993. 4. Patent RU 2005539, 1992. 5. B.N.Maksimov, V.G.Barabanov, Zhurn. prikl. Khimii, [Russ. J. Appl. Chem. (Engl. Transl.)], 1999, 72, v. 12, 1944-1948 6. Kijowski J., Webb G., Winfield J.M. J. Fluorine Chem., 1986, 27, 181-193 7. I.G. Trukshin, V.M. Vishnyakov, I.E. Mitina, S.G. Fedorova, T.E. Kramerova: Tez. dokl. Mezhdunar. semin. «Novye holodil'nye sistemy», (Sankt-Peterburg, 1995 g.), Sankt-Peterburg, 1995. 8. I.G. Trukshin. Kataliticheskie protsessy sinteza ftororganicheskih soedinenij. Kurs povysheniya kvalifikatsii po katalizu-II. Sbornik lektsij. Chast' II, Moskva, 2003, s.293-335. 9. G.P. Gambaretto, F. Avezzu and E. Gola J. Appl. Chem. Biotechnol., 1973, 23, 175-181 10. L. Kolditz, V. Calov, G. Kauschka und W. Schmidt, Z. anorg. allg. Chem., 1977, 434, 55-62 11. A.A. Bezdenezhnyh, Inzhenernye metody sostavleniya uravnenij skorostej reaktsij i rascheta kineticheskih konstant. Khimiya, Leningrad, 1973, 256 s. 12. A.V.Dmitriev, I.G.Trukshin, P.Yu.Smykalov Zhurn. prikl. khimii, [Russ. J.Appl.Chem. (Engl.Transl.)] 2002, 75, v. 5, s. 789-794 13. I.G. Trukshin, A.V. Dmitriev, A.S. Kuznetsov, V.G. Barabanov Tez. Dokl. 2-oj Mezhdunar. konf. CTAF’97 “Khimiya, tekhnologiya i primenenie ftorsoedinenij”, (Sankt-Peterburg, 1997) Teza, Sankt-Peterburg 1997, s. 39 14. Patent RU №2049085, 1993. 15. Patent RU №2124493, 1997. 16. Patent RU №1825485, 1992. 17. V.G. Barabanov, B.L. Nikiforov J. Fluorine Chem., 1999, 96, iss.1, p. 7-10 |
Fluorine Notes, 2009, 66, 1-2