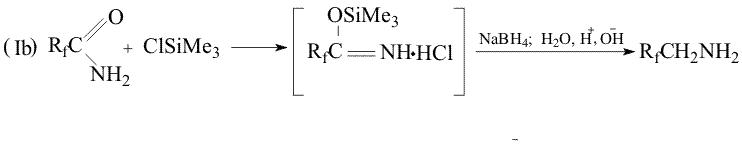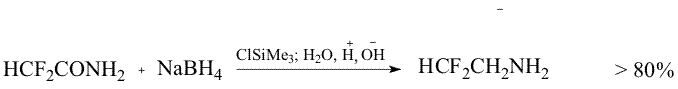Fluorine Notes, 2009, 65, 9-10
1,1-DIHYDROPERFLUOROALKYL AMINES. Report (II)
V.A.Vyazkov, A.F.Gontar, V.K.Grinevskaya, E.V.Igoumnova, S.M.Igoumnov
The establishment of Russian Academy of Sciences A.N.Nesmeyanov
Institute of Organoelement Compounds RAS e-mail: gontar@ineos.ac.ru
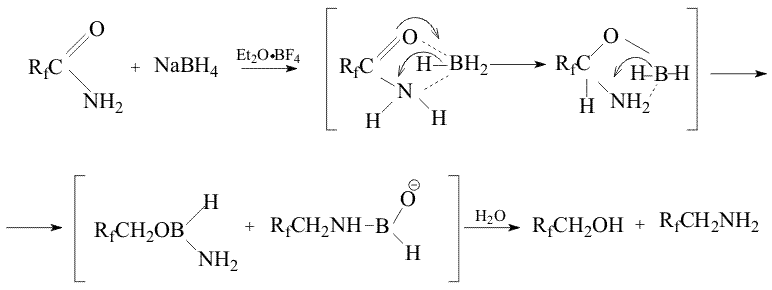
Reduction of primary amides of acids with borane (BH3), generated in Situ by addition of Et2O BF3 to the mixture of the amide and NaBH4 in an aprotic solution is well known [1,2]. We have shown earlier [3] that the reaction results in a mixture of corresponding primary amine and alcohol.
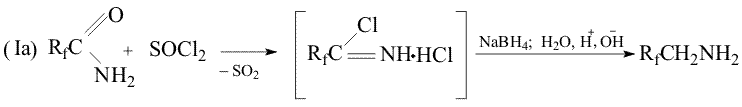
Throughout the research a new method has been developed, that makes possible the reduction of primary amides of perfluoroacids to amines excluding formation of alcohols. The method consists in treatment of the mixture of the amide and NaBH4 in an aprotic solution with either thionyl chloride or chlorotrimethylsilane. Probably in the first case (SОCl2) the formation of imidoylchloride takes place and in the second case (Me3SiCl) silyl ether of aminoacid is formed. As a result, in the first case oxygen is absent in the initial molecule that excludes a possibility of alcohol formation, in the second case oxygen is bound with silicon that suppresses the process of alcohol formation. The use of such a system results in a yield increase of the desired amine up to 80% apparently at the expense of suppression of the competitive reaction of the alcohol formation.
The latter variant was scaled by us in production of 1,1dihydrodifluoroethylamine up to 10.0 kg in one batch.
Experimental Method Ia Sodium boron hydride (5.07g, 0.15 mol) was added to the solution of difluoroacetamide (9.5g, 0.mol) in 30ml of dioxane at 20oC and thionyl chloride (18g, 015 mol) was added dropwise. The reaction mass was stirred for 30 min, heated up to 100oC and kept for 1 hour, then cooled to 50oC and the solution of NaOH (8g) in 8 mL water was added dropwise ( with simultaneous distillation of the desired product boiling before 70oC) . There was obtained 5.05 g of the distillate with the product content of 67% (Gas liquid chromatography) Method Ib Sodium boron hydride (3 kg, 75 mol) was added at stirring to the solution of difluoroacetamide (6kg, 63 mol) in dioxane(15.8 kg).Trimethylchlorosilane (7.6 kg, 68 mol) was adding to the obtained suspension for 1.5-2 hours keeping the temperature of the reaction mixture stable. Then the temperature was gradually increased to 90-95oC and the reaction mixture was kept for another 5-6hours, then cooled to 75-80oC and the solution of NaOH (5kg) in 5 dm3 of water was added at such a rate that the raw would be distilled evenly. The product residue was distilled by heating the reactor to 100-105oC. There was obtained 12.4 kg of the raw with the product content of ~35%. After rectification there was produced 3.8 kg of the amine with BP= 68-63oC in 84% yield, 97% + purity. References
|
Fluorine Notes, 2009, 65, 9-10