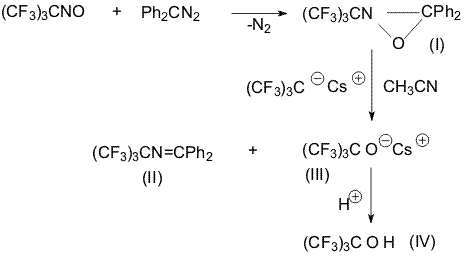Fluorine Notes, 2009, 65, 7-8
Interaction of 2-perfluoro-tert-butyl-3,3-diphenyloxaziridine with perfluoro-tert-butyl anioneD.P.Del'tsova
The establishment of Russian Academy of Sciences A.N.Nesmeyanov
Institute of Organoelement Compounds RAS, It was shown earlier that oxazaridine (I) obtained from 2-nitrosoperfluoroisobutane and diphenyldiazomethane possessed properties both as weak electrophile and as weak nucleophile [1]. It enters cycloaddition reactions at 50-60oC with such strong electrophiles as perfluorobutylisocyanate, hexafluoroacetone and bis(trifluoromethyl)ketene. Perfluoroisobutylene does not react with oxaziridine under the same conditions [1]. But in the presence of CsF in acetonitrile at 20oC there are formed imine (II) and cesium salt of perfluoro-tert-butanol (III) from which perfluoro-tert-butanol was produced (IV) [2,3]. Under the action of CsF perfluoroisobutylene is obviously converted into highly nucleophilic perfluoro-tert-butyl anion that attacks the oxaziridine ring. Further conversions bring to the formation of imine (II) and cesium salt of perfluoro-tert-butanol (III). In fact, oxaziridine oxidizes perfluoro-tert-butyl anion reducing to the imine. An attempt to obtain fluorine-containing α-lactone at interaction of oxaziridine(I) with perfluorodimethylketene in the presence of CsF brought to the formation of a non-identified mixture of products and in the case of N-phenyl-bis(trifluoromethyl)ketenimine there was obtained a dimmer of the latter [4]. Experimental Perfluoroisobutylene ( 2 mL) was passed into a suspension of freshly calcined CsF( 1.5g, 0.01mol) in 10 Ml of abs. acetonitrile, the mixture was stirred for 2 hours at 20oC and the solution of oxaziridine (I) (4.1g, 0.01mol) in 10 ml of abs. acetonitrile was added dropwise. The reaction mixture was stirred for 48 hours at 20oC, the volatile products were distilled into a trap under vacuum. Absolute ether was added to the residue, the deposit was filtered. There was obtained 3g ( 81%) of Cs-salt of perfluoro-tert-butanol (III) that was dissolved in water, sulfur acid was added to reach pH2, the bottom layer was separated and distilled over concentrated sulfur acid. There was obtained 1.5g of perfluoro-tert-butanol (IV), BP= 45-47oC. After distillation of the ether from the filtrate the residue was distilled. There was obtained 2.8g (70%) of imine (II), BP=82-84oC/ 0.01 mm Hg. References 1. Del'tsova D.P., Gambaryan N.P., Lur'e E.P., Izv. AN SSSR, Ser. khim. 1979, N 8, 1788. 2. Zeifman Yu.V., Izv. AN SSSR, Ser. khim.,1992, N 2, 464. 3. Stefan P. Kotun, John D. O. Anderson, and Darryl D. Des Marteau, J. Org. Chem., 1992, 57 (4), 1124-1131. 4. Del'tsova D.P., Gambaryan N.P., Zeifman Yu.V., Knunyants I.L., Zh. Org. Khim., 8, 856, (1972). |
|
|
Fluorine Notes, 2009, 65, 7-8