Fluorine Notes, 2009, 65, 5-6
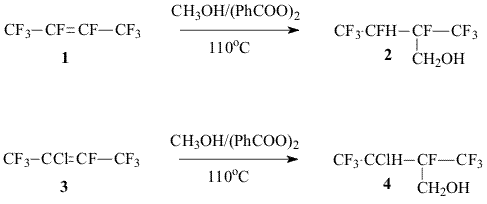
2-TRIFLUOROMETHYL-3,3,4,4,4-PENTAFLUOROBUTENE-1 SYNTHESISE. A.Avetisyan, V.F.Cherstkov Institution of Russian Academy of Sciences A.N.Nesmeyanov Institute of Organoelement Compounds RAS, Russian Federation, 119991 Moscow, Vavilova st. 28 Hexafluoroisobutylene and its derivatives are used for production of polymers. It is known that non-symmetrical hexafluoroisobutylene analogues are of interest for synthesis of amorphous polymers [1]. We have developed a new approach to the synthesis of its homologue, 2-trifluoromethyl-3,3,4,4,4-pentafluorobutene-1 (8), on the basis of available fluorobutenes including the following stages:. a) Radical addition of methanol to octafluorobutene-2 (1) or to heptafluoro-2-chlorobutene-2 (3) [2].
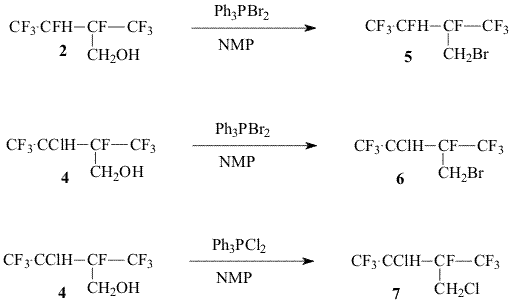
b) Replacement of the hydroxyl group for halogen
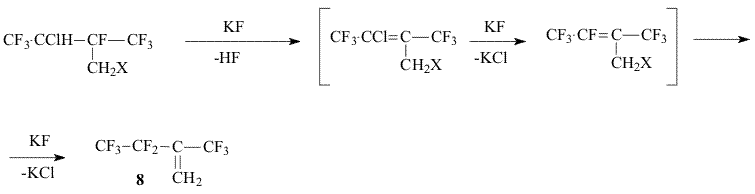
c) Treatment of the obtained halides (5,6,7) with potassium or cesium fluorides in a polar solvent at 100-150°C followed by dehydrohalogenation, substitution of the allyl chlorine or bromine for fluorine and isomerization of obtained olefin 8.
Earlier [3] we proposed to convert alcohols (2,4) into corresponding chlorosulfites, but the reaction with thionyl chloride is accompanied by the formation of a considerable share of symmetrical dialkylsulfites that reduce significantly the total yield of target olefin 8. In the present work for exchange of the hydroxyl group for halogen we used either triphenylphosphine dichloride and triphenylphosphine bromide in dimethylformamide or N-methylpyrrolidone that made possible to afford chloride 7 and bromides 5,6 in a high yield. Experimental The 19F NMR and 1H NMR spectra were recorded on a “Bruker AC-200 X” instrument ( 200 Hz for 1H and 188.3 Hz for 19F accordingly, TMS and CF3COOH standard, chemical shifts in ppm, spin-spin coupling constants in Hz).The mass-spectra were recorded on a V6-7070F instrument at ionizing irradiation energy of 70eV. For the reaction there were used dry N-methylpyrrolidone, CsF. 1,1,3-Trihydro-3-chloroperfluoro-2-methylbutanol-1 (4) a.The mixture of 2-chloroperfluorobutene-2 (3)(28g, 0.12 mol) , methanol (30 mL) and benzoyl peroxide(BP) (0.8g) was heated in a steel autoclave at a temperature of 110oC for 10 hours. By distillation of the reaction mixture there was obtained 18.4 g of carbinol (4) with the boiling temperature of 138-143oC ( two diastereomers) Found: C 24.38; H 1.63%. C5H4ClF7O. Calculated: C 24.14; H 1.61%. 19F NMR: -8.1 m and -8.2 m (CF3); -1.8 m and -2.0 m (CF3); 99.2 v (CF). b. The mixture of 2-chloroperfluorobutene-2 (22g, 0.1 mol), methanol (20 mL) and BP (0.3g) was heated at a temperature of 110oC for 6 hours in a sealed glass ampoule. After removal of BP residue and methanol excess, by distillation there was obtained 24.8 g (97%) of carbinol 4. Br2 (8g, 0.05 mol) was added to triphenylphosphine (13.1g, 0.05 mol) in 15 mL of N-methylpyrrolidone. After completion of the exothermal reaction carbinol (2) (10g, 0.043mol) was added dropwise. The reaction mixture was heated at stirring at a temperature 120-140oC slowly distilling low-boiling products. There was obtained 7.8 g of bromide 5 with the boiling temperature of 97-98,5oC ( two diastereomers). The mass spectrum (m/z, rel. intensity, %):294[M]+ 45; 274 [M-HF]+64; 255 [M-F-HF]+2; 225 [M-CF3]+ 3; 213 [M-HBr]+ 4; 205 [M-HF-CF3]+15; 195 [M-Br-HF]+ 55; 145 [CF2C(CF3)=CF2]+ 73; 111[CBrHF]+ 32; 93 [CH2Br]+ 100; 69 [CF3]+ 92; 51 [CF2H]+ 68.. 19FNMR: -2.5 m and -1.3 m (CF3); 99.5 m and 100.8m (F 1); 136 m and 1366 m (F2) 1H NMR: 4.2 m (CH); 2.6 mì (CH2). 2,4,4-Trihydro-3-chloro-4-bromoperfluoro-3-methylbutane (6) Bromide (6) was obtained similarly to bromide (5) from carbinol (4) as two diastereomers, Tb= 128-132oC, in 70% yield. 19FNMR: -3.2m and -7.5m (CF3); 90.2m (CF). 1H NMR: 3.6m (CH); 2.5m (CH2). 2,4,4-Trihydro-3,4-dichloroperfluoro-3-methylbutane (7) Chloride (7) was obtained similarly to bromide (6) by addition of carbinol (4) to the reaction mixture of equimolar quantities of triphenylphosphine, Cl2 in N-methylpyrrolidone. The mass spectrum ((m/z, rel.intensity, %): 266[M]+5; 246 [M-HF]+32; 230 [M-HCl]+ 2; 211 [M-HF-Cl]+9; 197 [M-CF3]+ 7; 177 [M-C2F5]+ 38; 69 [CF3]+ 65; 49 [CH2Cl]+ 100.. 19F NMR: -8.5m and -3.1m (CF3); 1HNMR : 3.8m (CH); 2.4m (CH2). 2-Trifluoromethyl-3,3,4,4,4-pentafluorobutene-1 (8) Bromide (5) (7.8g, 0.025 mol) was added dropwise to the solution of CsF (8g, 0.05mol) in 15 mL of N-methylpyrrolidone. The reaction mixture was heated at stirring to 95-100oC, distilling olefin (8) into a cold trap connected to a water-jet pump ( ~100 mm Hg). By distillation there was produced 3.8g (71%) of olefin(8) identical to [3]. In a similar way olefin (8) was produced from bromide (6) and chloride (7). Literature 1. R. H. French, R. C. Wheland, W. Qiu, M.F. Lemon, E. Zhang, J. Gordon, V. A. Penrov, V. F. Cherstkov, N. I. Delyagina, J. Fluorine Chem.2003, 122, 63-80 2. J. D. Lazerte, R. J. Koshar, J.Am. Chem. Soc., 1955, 77, 910 3. V. A. Petrov, W. J. Marshall, C. G. Krespan, V. F. Cherstkov and E. A. Avetisian , J. Fluor.Chem. , 2004, 125, 99-105 |
Fluorine Notes, 2009, 65, 5-6