Fluorine Notes, 2009, 65, 3-4
The Reactions of 1,2-bis(fluorosulfonyloxy)tetrafluoroethane with Nucleophiles
N.I.Delyagina, V.F.Cherstkov, S.R.Sterlin*
The establishment of Russian Academy of Sciences A.N.Nesmeyanov Institute of Organoelement Compounds RAS
Russian Federation, 119991 Moscow, Vavilova st. 28
Fax: +7(499) 135 6549, E-mail:lsg@ineos.ac.ru
The perfluoroalkylfluorosulfates are cleaved under the action of nucleophilic agents with the formation
of carbonyl compounds – perfluoroacyl fluorides or ketones1-4. In contrast
to their saturated analogues the reactions of nucleophiles with fluorinated aliphatic and aralkyl
fluorosulfates, where FSO3-group is located in  -position
towards sp2-hybridized C-atom in the composition of vinyl or carbonyl group, afford the products
of substitution of FSO3-group for nucleophilic residue5-7.
-position
towards sp2-hybridized C-atom in the composition of vinyl or carbonyl group, afford the products
of substitution of FSO3-group for nucleophilic residue5-7.
Thus, it was shown7 that the reactions of ethyl fluorosulfonyloxydifluoroacetate (1) with soft nucleophiles give substituted ethyl difluoroacetates RCF2 COOEt (2) (R = Br, I, PhS, i-C3F7O).
The ester 1 was obtained by alcoholysis of fluorosulfonyloxydifluoroacetyl fluoride (3) but the source of acetylfluoride 3 hadn’t been indicated*. In the given work we have shown that the ester 1 is formed smoothly by alcoholysis of 1,2-bis(fluorosulfonyloxy)tetrafluoroethane (4) in the presence of NaF. Bis-fluorosulfate 4 is available by anodic oxidation of tetrafluoroethelene in HSO3F/[KOSO2F]8.
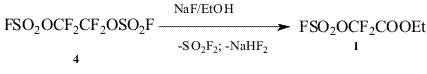
It should be noted that esters 2 can be prepared directly from bis-fluorosulfate 4. Thus, the reaction of 4 with LiCl in diglime leads to the formation of chlorodifluoroacetyl chloride (5) that was characterized in the form of ester 2 (R = Cl) (2a).
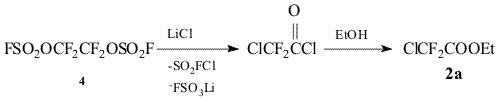
Ethyl fluorosulfonyloxydifluoroacetate
Bis-fluorosulfate 4 (15 g, 50 mmol) was gradually added to suspension of NaF (2.7 g, 65 mmol) in 25 ml of abs. EtOH, after gas evolution ceased the reaction mixture was stirred for 30 min, filtered, washed with diluted HCl acid, the organic layer was separated, dried over MgSO4 and distilled to give 9.1 g (82%) of ester 1, b.p. 74-76º/100 Torr (lit. data7: b.p. 75-76o/100 Torr). NMR 19F spectrum was identical to that described in7.
Ethyl Chlorodifluoroacetate
Bis-fluorosulfate 4 (20g, 67 mmol) was gradually added to solution of 9.45g (225 mmol) of LiCl in 50 ml of dry diglime, the reaction mixture was stirred at 55-60o/5 h, the volatile products evolved were passed through abs. EtOH at ~0o, after the reaction was completed the alcoholic solution was washed with cold water, organic layer was separated, dried over MgSO4 and distilled to give 6.9 g (65%) of ester 2a, b.p. 96-98o (lit. Data: b.p. 97o)9.
Literature
1. Lustig M., Ruff J.K., Inorg. Chem. 1964. V. 3. P. 287; ibid. 1965. V. 4. P. 1441
2. Shack C.J., Christe K.O., ibid. 1982. V. 21. P. 283
3. Krespan C.G., J.Fluor. Chem. 1972. V. 2. P. 173
4. Kurykin M.A., German L.S., Studnev Yu.N., Fokin A.V., Izv. Acad. Sci. USSR, Ser. chim., 1980, vol. 7, P. 1679
5. Cherstkov V.F., Sterlin S.R., German L.S., Knunyants I.L., Izv. Acad. Sci. USSR, Ser.chim., 1982, vol. 12, P. 2791
6. German L.S., Knunyants I.L., Sterlin S.R., Cherstkov V.F Izv. Acad. Sci. USSR, Ser.chim., 1981, vol. 8, P. 1933
7. German L.S., Mukhametshin F.M., Delyagina N.I., Korovushkin G.G., Izv. Acad. Sci. USSR, Ser.chim., 1985, vol.6, P. 1435
8. Rogovik V.M., Cherstkov V.F., Grinberg V.F., Vasil’ev Yu.B., Peterleytner M.G., Sterlin S.R., German L.S., Izv. Acad. Sci. USSR, Ser.chim.,1991, vol. 10, P. 2362
9. Ray P.C., Ray A.C., J. Indian Chem. Soc., 1936, V. 13, P. 427
Fluorine Notes, 2009, 65, 3-4
