Fluorine Notes, 2009, 64, 13-14
The Interaction of Polyfluorinated β-diimines And Perfluorocarboxylic Acids' FluoroanhydridesD. U. Verbitsky, * M. A. Kurikin A.N.Nesmeyanov Institute of Organoelements
Compounds RAS. 119991, Vavilova str.28, Moscow, Russia, The tris-(perfluoroalkyl)pyrimidines synthesis methods based on the reaction of polyfluorinated β-diimines and perfluorocarboxylic acids fluoroanhydrides has been developed The condensation of polyfluorinated β-diimines (1) with acylhalogenides resulting in forming of 2,4,6-trisubstituted pyrimidines [1] opens wide opportunities for synthesis of new biologically active compounds and monomers as well. Before [2] it was proved, that at interaction with perfluorocarboxylic acids' anhydrides and chloroanhydrides the diimines (1) form 2,4,6-tris-(perfluoroalkyl)-5-fluoropyrimidines (2). 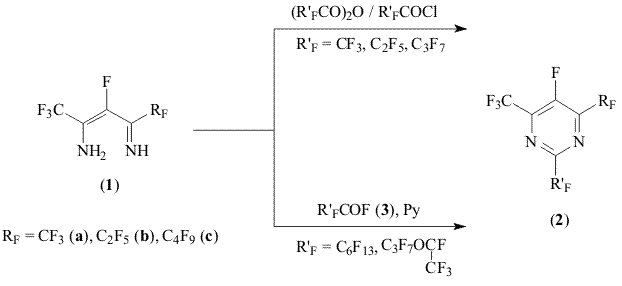
The distribution of this reaction over industrially available perfluorocarboxylic acids fluoroanhydrides (3) is of obvious interest from the practical point of view. The present work is dedicated to the topic. The β-diimines (1) appeared to interact with fluoroanhydrides (3) at room temperature in the solution of absolute diethyl ether. However the yields of isolated required pyrimidines (2) appeared to be very low (~40%). Along with that the products of acidic decomposition of initial diimine (for RF = CF3 - tetrafluoroaceton and trifluoroacetic acid) were detected in the reaction mass. The condensation discussed in this review is followed by the isolation of by-products
- water and acid, which interaction with the initial diimine (1) results
in its destruction. This process is likely to be put into practice in case
of using any of acylating reagent (anhydride, chloro- or fluoroanhydride).
If condensation is being realized in a quick way (anhydride, chloroanhydride),
then the decomposition of In case of fluoroanhydrides (3) differing from corresponding chloroanhydrides by their lowered reactivity in analogous processes [3], the acidic decomposition of β-diimines (1) starts to compete with the main reaction. Indeed, when carrying out the condensation of diimines (1) and fluoroanhydrides (3) over acid acceptor (pyridine) the side process associated with the hydrolysis of compounds (1) was managed to put down and pyrimidines (2) were isolated with preparatory yields (up to 87%). Experimental NMR 19F spectra were registered at "Bruker AC-200F" spectrometer (188,3 MHz). Chemical shifts are given in ppm with CF3COOH as standard, the coupling constants reported in Hz. Perfluoro-2-hexyl-4-butyl-6-methylpyrimidine (2c, R'F = n-C6F13) 1. In the absence of pyrimidine. 2. Over pyrimidine. Analogously other perfluoropyrimidines were synthesized (2); the details of experiments as well as the spectrum information and physical constants of obtained compounds are listed in Table 1. References 1. O.E. Petrova. Diss. kand. khim. nauk, INEOS RAS, Moskva, 2003, 122s. 2. O. E. Petrova, M.A. Kurykin, D.V. Gorlov, Izv. AN, Ser. Khim.‚ 1999, N 11, 2195. 3. N.O. Calloway, J. Am. Chem. Soc., 1937, 59, 1474. 
Table 1.
|
Fluorine Notes, 2009, 64, 13-14
