Fluorine Notes, 2009, 64, 11-12
Fluorine-containing pyrazoles
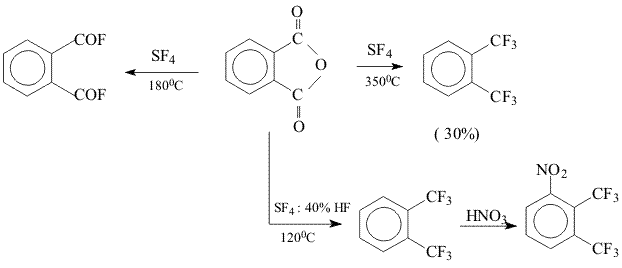
A.F.Gontar A.N.Nesmeyanov Institute of Organoelements Compounds RAS. 119991, Vavilova str.28, Moscow, Russia It has been shown earlier that for production of 1,2-bis (trifluoromethyl) benzene from phtalic anhydride a temperature up to 350 oC is necessary [1,2].
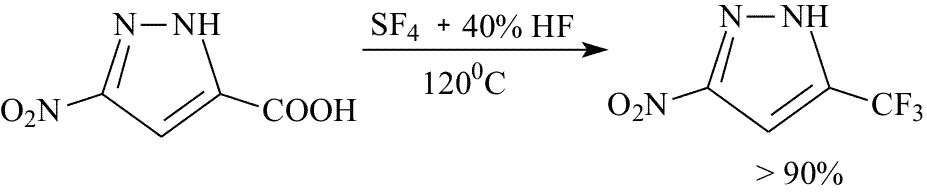
During the process of development of a method to produce 2,3-bis(trifluoromethyl)nitrobenzene we succeeded in a considerable decrease in the reaction temperature by addition of a small quantity of hydrofluorice acid (40% solution of HF in H2O) into the reaction mixture. It turned out that the use of SF4 with addition of hydrofluoric acid could be successfully used for substitution of carboxyl group in pyrazoles. Thus, by treatment of 3-carboxy-5-nitropyrazole with this mixture a corresponding pyrazole containing trifluoromethyl group was synthesized in a yield up to 90%.
Experimental 1,2-Bis(trifluoromethyl)benzene 3-trifluoromethyl-5-nitropyrazole Found,%: C, 26.32; H, 3.40; N, 22.42 C4H6N3O4, References 1. A.P.Khardin, B.N.Gorbunov, P.A.Protopopov, Chemistry of sulfur tetrafluoride, Ed. Saratov University, 1973, p.123 2. W.R. Hasek, W.C. Smilh., V.A. Engelhardt . I. Aw. Chem. Soc.,82,543 (1960) |
Fluorine Notes, 2009, 64, 11-12