Fluorine Notes, 2009, 64, 9-10
4-(PERFLUOROALKYL)IODBUTANES
|
|
RF(CH2)4I |
BP ( oC) |
Conversion, % |
Yield, % |
|
CF3 (a) C2F5 (b) C3F7 (c) C3F7 (d) C4F9 (e) C6F13 (f) C8F17 (g) cyclo-C6F5 (i) |
53/12 mm Hg 62-64/15 mm Hg 115/100mm Hg 72/15 mm Hg 96-97/25 mm Hg 28-30 (MP) 50-51 (MP) 93/9 mm Hg |
50 54 60 64 50 42 40 48 |
71 63 58 55 75 32 35 38 |
The obtained 4-(perfluoroalkyl)iodobutanes (IIa-b) may be converted to corresponding alcohols.
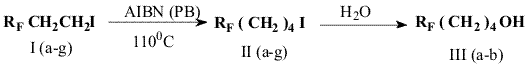
Experimental
5-Iodo-1,1,1-trifluoropentane (IIa)
3-Iodo-1,1,1-trifluoropropane (200g,
0.89mol) and azobisisobutyronitrile (AIBN, 3 g) are fed in a steel
rotating autoclave (0.5L water capacity) equipped with a needle valve.
The autoclave is pressurized, ethylene is fed to attain a pressure of 45
atm, the autoclave is heated to a temperature of 110oC and kept for
another 4 hours. Then it is cooled to room temperature, the ethylene
excess is released through the needle valve and the liquid is discharged
in a flask. The non-reacted 3-iodo-1,1,1-trifluoropentane is distilled
from the reaction mass at a boiling temperature of 90-95oC (80g), the
residue is distilled under vacuum at a boiling temperature of 50oC/10
mm Hg collecting fractions. 90g of the crude material is collected.
After rectification 80g of 5-iodo-1,1,1-trifluoropentane (IIa) of 97%
purity remains, BP 153oC, 71% yield, 50% conversion. Another
4-(perfluoroalkyl)iodobutanes (II b-i) are obtained in a similar way (
see Table 1).
5,5,5-Trifluoropropenpentan-1-ol (IIIa)
N-Methylpyrrolidone (500mL),
water(30g, 1.66 mol) and 5-iodo-1,1,1-trifluoropentane (175g, 0.69 mol)
are placed in a three-neck flask ( 1 L water capacity) fitted with a
thermometer, stirrer and backflow condenser. The reaction mass is heated
up to 120oC and mixed at this temperature for 20 hours. After cooling
the reaction mass to room temperature the backflow condenser is replaced
for a direct one and all volatile products are distilled in vacuum of a
water-jet pump. The distillate is poured into 1L of 15% hydrochloric
acid. The obtained solution is extracted with ether (3X250mL). Ether
extracts are dried over MgSO4 , ether is distilled, the residue is
distilled under vacuum to yield 50g of crude product containing 80% of
5,5,5-trifluoropentan-1-ol. After rectification 30g of
5,5,5-trifluoropentan-1-ol (IIIa) of 99% purity is produced in 30% yield
, BP 73oC/ 30 mm Hg.
5,5,6,6,6-Pentafluorohexan-1-ol (III b)
6-Iodo-1,1,1,2,2,2-pentafluorohexane (183g,0.61 mol),
N-methylpyrrolidone (780 mL) and water ( 34g, 1.89mol) are placed in a
three-neck flask ( 2 L water capacity) fitted with a mechanical stirrer,
thermometer and a backflow condenser. The mixture is heated up to a
temperature of 140-145oC and is stirred for 20 hours at this
temperature. Then the backflow condenser is replaced by a direct one,
the organic layer is separated, washed with water, dried over MgSO4 and
distilled. There is obtained 20 g of
6-iodo-1,1,1,2,2,2-pentafluorohexane that is returned to the synthesis
and 65g of 5,5,6,6,6-pentafluorohexan-1-ol ( 30g of 93% purity and 35 g
of 96.5% purity). The yield is 63% ( taken into account conversion).
References
1. W.R. Dolbier. Chem. Rev. 1996,96,1557-1584.
2. N.O. Brace. Journal of Fluor. Chem., 93 (1999) 1-25.
Fluorine Notes, 2009, 64, 9-10
