PERFLUOROALKYLISONITRILES
A.F.Gontar
A.N.Nesmeyanov Institute of Organoelements
Compounds RAS. 119991, Vavilova str.28, Moscow, Russia.
e-mail:
gontar@ineos.ac.ru
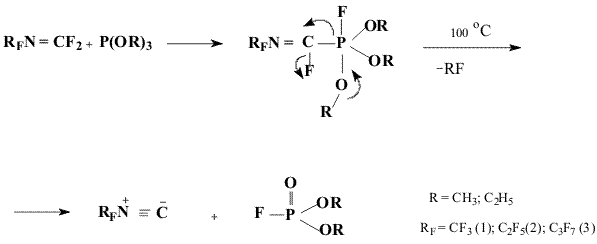
It has been found that the reaction of fluoroazaalkenes (RfN=CF2)
with trialkylphosphites brings to synthesis of adducts- trialkylperfluoroalkylfluorophosphoranes
that may be used without isolation in individual form for production of perfluoroalkylisonitriles
(1-3) unavailable earlier.
Experimental
Trifluoromethylisonitrile CF3- N=C ( 1)
Trimethylphosphite
(12.4 g, 0.1 mol) is placed in a four-neck flask fitted with a thermometer,
stirrer, gas supply and gas outlet pipes connected with a cold trap cooled
to -120oC. The flask is cooled to a temperature of -40oC
with a mixture of acetone- solid carbon dioxide and perfluoro-2-azapropene
(13.3g,0.1mol) let pass at stirring. Then cooling is stopped and after the
reaction mass reaches room temperature the flask is heated on a boiling water
bath to 85-90oC.
The entrapped products are fractionated in a low temperature still. There is
produced 7.5g of trifluoromethylisonitrile, boiling temperature from -83
to -81oC. The yield is 79%.
Found,%: C, 24.93; N ,14.52;
MWt 94.8
C2NF3 Calculated,%: ?, 25.26; N ,14.73;MWt
95.0
Pentafluoroethylisonitrile C2F5N=C (2)
Analogously to (1) from trimethylphosphite (12.4g, 0.1 mol) and perfluoro-2-azabut-1ene
(18.3g, 0.1 mol) there is produced pentafluoroethylisonitrile (10.6g); BP
-42oC. The yield is 73.2%.
Found, %: C, 25.13, N, 9.57; MW
143.8.
C3NF5 Calculated, %: C, 24.83; N, 9.66,
MW 145.0.
Heptafluoropropylisonitrile C3F7N=C (3)
Similar to (1) from trimethylphosphite (12.4g, 0.1 mol) and perfluoroaza-2-pent-1-ene
there is produced perfluoropropylisonitrile (15.9g); BP -25 oC.The
yield is 70.9%.
Found, %: C, 24.13; N, 7.29; MW 196.3
C4NF7 Calculated,
%: C, 24.62; N, 7.18; MW 195
References
1. Makarov
S.P.‚ Englin M.A., ZhOKh, 1967, t. 37‚ s. 2781
2. Banks
B.E., Hasseldine R.N., J. Chem. Soc. 1969, (C), p. 2119, |