NUCLEOPHILIC ISOMERISATION OF HEXAFLUOROPENE OXIDE
A.F.Gontar
A.N.Nesmeyanov Institute of Organoelements
Compounds RAS. 119991, Vavilova str.28, Moscow, Russia.
e-mail:
gontar@ineos.ac.ru
Attempts to obtain pentafluoropropionyl fluoride (PPF) undertaken earlier [1-8]
, except for the synthesis method of this compound by the action of Yarovenko
reagent on pentafluoropropionic acid [9], have no preparative importance.
With the goal to develop a convenient method to obtain PPF in the present work
we studied "nucleophilic" isomerization of hexafluoropropene oxide (HFPO).
The latter is an available product of fluoroorganic manufacture.
We studied the action of various reagents (KF, CsF, pyridine, triethylamine and
other bases) on HFPO both in a flow system by passing through a heated pipe
filled with fluorides of alkali metals and by bubbling HFPO through a base
solutions (pyridine, triethylamine, quinoline) followed by capturing the
forming products.
As the result of these experiments it has been found that variations of HFPO
flow rate, temperature, time of interaction and other process parameters
do not allow to attain full conversion of HFPO into PPF and as the boiling
temperatures of these substances are very close (~-28 o C) it is impossible
to isolate PPF in pure form.
At the same time it was found that full HFPO isomerization takes place in
a close system under the action of quinoline.
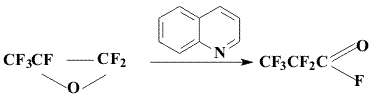
The found method is extremely convenient and allows producing PPF in great
quantities.
Experimental
Quinoline (200g, 1.55 mol) and hexafluoropropylene oxide (2400g, 14.46 mol)
are placed in a steel autoclave ( 2L water capacity) with a needle valve
cooled to a temperature of -70 oC. The autoclave is hermetically
sealed and heated. At a temperature of 10oC there is observed
a temperature jump up to 60oC in the autoclave.
The autoclave is placed in a rocking furnace, the temperature is raised to
100 oC and kept for 2 hours. Then the autoclave is cooled
to room temperature, the product is collected through the needle valve
in a trap cooled with dry ice. There was obtained 2200g of Hexafluoropropionyl
fluoride of 97% purity in 92% yield, BP=-28oC.
19F
NMR: 8.0 ppm (CF3), 46 ppm ( CF2), -98 ppm (COF)
References
1. Cox D.G., Sprague L.C.,
Burton D.G., J. Fluor Chem. 23, 1983, 383-388 2. Pallerite M.J., J. Fluor
Chem. 49, 1, 1990, 43-66 3. Ruff M., J. Org. Chem, 30,
1965, 3968 4. Gubanov B.A., Tyul'sa G.M.,
Solodkaya I.G.‚ SHerman M.A., ZhOrKh, 1984, t. 20‚ N 3, s. 493-498 5. Berenblit V.V.‚ Byzov B.A.,
Dolgopol'skij I.M.‚ Dolnakov Yu.P., ZhPKh, 1974, t. 47, N 11, s. 2433-2435 6. Berenblit V.V.‚ Byzov B.A.,
Dolgopol'skij I.M.‚ Dolnakov Yu.P., Grachev V.I.‚ ZhPKh, 1975, t. 48, N 3, s.
709-711 7. Sianes D., Sasette A.‚
Tarli F., J. Org. Chem, 31, 1966, 2312 8. Knunyants I.L., Shokina
V.V., Galakhov I.V., Khim. Geteroc. S. 1969, 873
9. Fokin A.B., Studnev YU.N‚
Rapkin A.I., Suptanbekov D.A., Potarina T.M., Izvestiya AN SSSR, 1984, N 2,
411-415
|