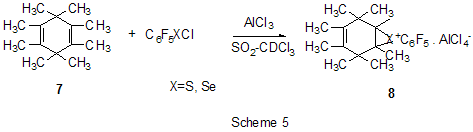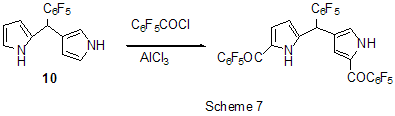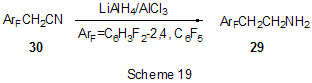Fluorine Notes, 2009, 63, 3-4
AlCl3 in modern chemistry of polyfluoroarenesT.D.Petrova, V.E.Platonov
Novosibirsk N.N. Vorozhtsov Institute of Organic Chemistry of SB
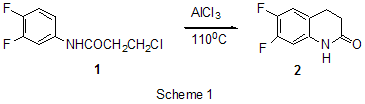
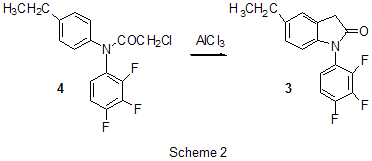
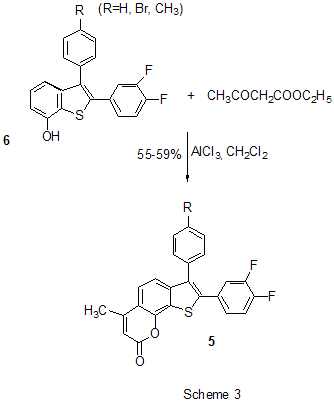
RAS, 630090, Novosibirsk, prospect Acad. Lavrentiev, 9 Summary AlCl3 is one of the most important and widely used catalysts known a long ago in laboratory and industrial practice. This review informs of AlCl3 application in a relatively new field of organic chemistry, chemistry of polyfluoroaromatic compounds, since 1995y. Conversions of aromatic compounds containing two and more fluorine atoms on one aromatic ring have been under consideration. Systematization was made according to the type of running conversions. The list of literature for the period under consideration is not exhaustive, but illustrates completely all directions of using AlCl3 in this field. Key words: AlCl3, catalysis , Friedel-Crafts alkylation, acylation, cyclization, intermediates Introduction Long ago AlCl3 has become a classic reagent in organic chemistry and its role as a catalyst in organic reactions promoted by Lewis acids is well known [1-3]. Chemical activity, accessibility and cheapness of aluminum chloride are of constant attention of researchers in different fields of chemistry. It was used in organofluorine chemistry as well [4,5]. Recently for acid-catalyzed reactions of different type compounds along with traditional mineral acids and Lewis acids new type catalysts were started to use, for example solid acids and solid super acids [6 and references], metal complexes (Hf, Sc, Ga, Bi, Yb) with -N(SO2C8F17)2 and -OSO2CF3 ligands, including Al(OSO2CF3)3 [7], as well as aluminum chlorofluorides of general formula AlClxFy [8-10]. Nevertheless, in spite of appearance of new catalytic reagents, the use of AlCl3 is continued. Chemistry of polyfluoroarenes is one of comparatively new fields in which possibility of using AlCl3 has been demonstrated. This report presents a data review of AlCl3 application in the chemistry of polyfluoroarenes beginning since 1995y. As substrates or reagents there are given fluorinated compounds containing two and more fluorine atoms in one aromatic ring and the transformations are systematized according to the type of the reactions taking place. 1. Friedel-Crafts alkylation and acylation reactions Among the reactions catalyzed by AlCl3, both in series of polyfluoroarenes and for non-fluorinated compounds the most common are Friedel-Crafts alkylation and acylation reactions. Substitution of hydrogen atoms for fluorine in the aromatic ring of polyfluoroarenes reduces reactivity of such compounds towards electophilic alkylating and acylating agents, but activation of these agents under the action of AlCl3 is sufficient for successful course of these reactions. At that a polyfluorinated compound may act both as a substrate and as a reagent and the process itself may proceed with participation of the polyfluorinated ring and with another molecule moiety as well. Thus, AlCl3 immobilized on a substrate was used for monoisopropylation of metha-difluorobenzene [11]. Chlorometylation of 1,3,5-trifluorobenzene in the presence of AlCl3 by the action of chloro(methoxy)methane in carbon bisulfide affords 1,3,5-tris-(chloromethyl)-2,4,6-trifluorobenzene in 80% yield [12]). The interaction of 1-bromo-2,3,5,6-tetrafluorobenzene with CHCl3 and AlCl3 results in tris(4-bromo-2,3,5,6-tetrafluorophenyl)methane in 22.4% yield [13]. The multi-stage synthesis of antibacterial agents of benzo[b]naphtiridones series includes the stage of intramolecular alkylation of 3-chloro-N-(3,4-difluorophenyl)-propionamide (1) in the presence of AlCl3 to give 6,7-difluoro-3,4-dihydro-1H-quinolin-2-one (2) in 78% yield [14](Scheme1). An example of the intramolecular alkylation reactions with cyclization in which course the polyfluorinated phenyl ring does not undergo transformations is obtaining 5-ethyl-1-(2,3,6-trifluorophenyl)-1,3-dihydroindol-2-one (3) from 2-chloro-N-(4-ethylphenyl)-N-(2,3,6-trifluorophenyl)acetamide (4) ( 48% yield) [15] (Scheme 2) and formation of 7,8-diaryl-4-methylcoumarinobenzo[b]thiophenes 5 in the interaction of 7-oxy-2-(3,4-difluorophenyl)-3-aryl-benzo[b]thiophenes 6 with acetoacetic ether and AlCl3 [16] (Scheme 3).
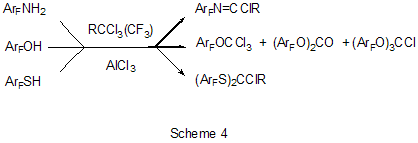
The reaction of polychloroalkylation of polyfluoroarenes that have N-,O- or S-containing functional groups and all the hydrogen atom on the ring are substituted for fluorine proceeds in a different way in comparison with above mentioned examples [17,18]. The substitution of hydrogen atoms for fluorine excludes the reaction of polychloroalkylation of the aromatic ring. Obviously, the transformation scheme includes the attack of donor heteroatom of the functional group by an electrophilic intermediate forming from polychloroalkylating agent under the action of AlCl3. Finally that results either in products of direct chloroalkylation, when such products are stable enough, or in products of their further transformation into compounds of different types (Scheme 4). S- and Se-substituted polyfluoroarenes themselves can interact with AlCl3 to give cationoid species in which the cationoid centers are localized on S or
Se atoms. Thus there has been postulated the formation of C6F5S+
and C6F5Se+ cations from C6F5XCl
(X=S, Se) and AlCl3, that with 1,2,3,3,4,5,6,6-octamethyl-1,4,-cyclohexadiene
(7) afford
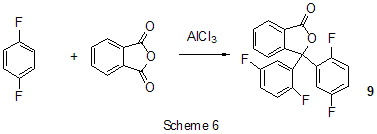
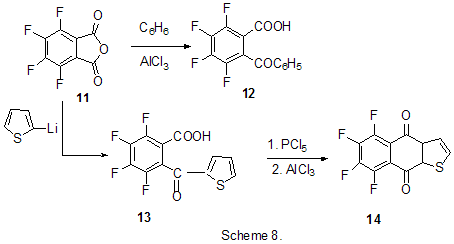
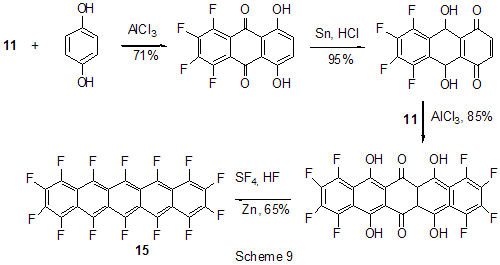
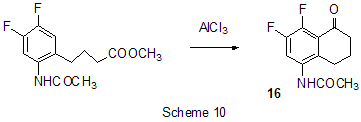
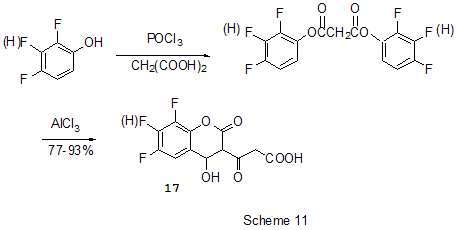
Friedel-Crafts acylation by the action of various acylating reagents is widely used in series of polyfluorinated benzenes to obtain appropriate polyfluorinated ketones in high yield. Thus, the acylation by acetylchloride of 1,2-difluorobenzene [20] and 4-chloro-1,2-difluorobenzene[21] results in formation of 1-(3,4-difluorophenyl)ethanone and 1-(2-chloro-4,5-difluorophnyl)ethanone. Acylation of 1,3-difluorobenzene with various acylating agents proceeds with the formation of 1-(2,4-difluorophenyl) keto-derivatives [20,22-32]. Unambiguously 2,4-difluorotoluene is acylated in position 5 [33], while substitution of a trimethylsilyl group in (3-chloro-2,6-difluorophenyl)trimethylsilane is used for synthesis of 1-(3-chloro-2,6-difluorophenyl)ethanone. The product yield is 54% [34]. The expected acylation products were obtained in reactions of 1-bromo-2,5-difluorobenzene [35] and 1,4-difluorobenzene [36-38], including phthalic anhydride as the acylation reagent [38] and its 4-bromo-derivative [36]. Surprisingly an attempt to synthesize 1,4-difluoro-10(dicyanomethylen)anthrone resulted in obtaining 3,3-bis(2,5-difluorophenyl)-3H-isobenzofuran-1-one(9) in the stage of interaction of 1,4-difluorobenzene with phthalic anhydride [39](Scheme 6). The acylation of 1,2,4-trifluorobenzene with acetyl chloride runs to 1-(2,4,5-trifluorophenyl)ethanone [20]. A similar process proceeds also in the case of 1,2,3-trifluoro2-methylbenzene when 1-(2,3,4-trifluoro-5-methylphenyl)ethanone is formed [40]. Analogously there were produced accordingly 1-(4,8-difluoronaphthalen-1yl)ethanone from 1,8-difluoronaphthalene [41], 1-(4,5-difluoronaphthalen-1-yl)ethanone from 1,8-difluoronaphthalene [42], 1-(1,3-difluoro-5,6,7,8-tetrahydro-5,5-dimethyl-2-naphthalenyl)ethanone from 5,7-difluoro-1,2,3,4-tetrahydro-1,1-dimethylnaphthalene [43]. The acylation of 1,3-difluorodiphenyl with succinic aldehyde led to the formation of 4-(2'4'-difluorobiphenyl-4-yl)-4-oxobutyric acid [44]. Polyfluoroarenes may act as acylating agents themselves. For example, 2,6-difluorobenzoyl chloride in the presence of AlCl3 acylates 1-benzosulfonylpyrrole to position 3 [45]. The acylation reaction of 3- methoxythiophene by 3,5-difluorobenzoyl chloride runs to position 2 along with demethylation of 3- methoxy group [46]. 2,4-Difluorophenylacetyl chloride acylates methylsulfide in position 4 [47], and pentafluorobenzoylchloride in the presence of AlCl3 allows insertion of the pentafluorobenzoyl group in the both pyrrole rings of pentafluorophenyl-substituted dipyrrolmethane 10 [48] (Scheme 7) and in position 5 in 2-bromothiophene [49] . Pentafluorophenyl ethers of Nα-substituted amino acids are used as acylating agents in interaction with free amino acids in peptide synthesis [50]. Aluminum chloride is used in this process as one of the component of AlCl3-DMF ( or another aprotic solvent)-pyridine system that promotes better solubility of the amino acid and correspondingly more complete reaction. The yield of di- and tripeptides under such conditions is over 90%. 3,6-Difluorophthalic anhydride in the reaction with para-xylene affords 2-(2,5-dimethylbenzoyl)-3,6-difluorobenzoic acid [51], and tetrafluorophthalic anhydride (11) reacts with benzene according to Friedel-Crafts to give 2-benzoyl-3,4,5,6-tetrafluorobenzoic acid(12) in 70% yield [52] (Scheme 8). Anhydride 11 was also used for the synthesis of 2-(2-thienylcarbonyl)-3,4,5,6-tetrafluorobenzoic acid (13). The intramolecular acylation of the latter under the action of PCl5 and AlCl3 brings to the formation of 5,6,7,8-tetrafluoro-4,9-dihydrohaphto[2,3-b]thiopen-4,9-dione (14) [52] (Scheme 8). Cyclization in the process of intramolecular acylation occurs also when anhydride 11 is used as the acylating agent in multi-stage synthesis of perfluoropentacene (15) which is of interest as a n-semiconductor for organic FET transistors [53,54] (Scheme 9). In production of novel liquid crystal materials of tetrahydronaphthalene structure there has been used a reaction of formation of 5,7-difluoro-3,4-dihydro-1H-naphtalen-2-one from ethylene and 3,5-difluorophenylacetic acid in the presence of AlCl3 [55]. Difluoronaphtoic acids which are used to obtain biologically active carboxamides have also been synthesized by Friedel-Crafts acylation and intramolecular cyclization of appropriate difluoroacetophenones and difluorophenylethylacetates[42]. The latter reaction was used for synthesis of difluoro-3,4-dihydronaphthalenones ( for example, 16 (Scheme 10), intermediates in the production of biologically active hexacyclic analogues of camptothecine [56]. Analogously, 2,4-difluorophenylpropionic acid was converted into 4,6-difluoro-1-indanone which is used for the synthesis of fluorinated 1-indanylidene acetamide, a potential miorelaxant of central action [57]. The intramolecular cyclization was also used in the synthesis of biologically active fluorinated 4-oxycoumarines 17 derived from available fluorinated phenols [58] (Scheme 11).
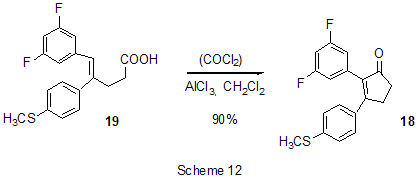
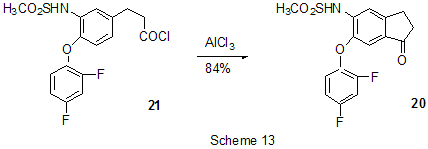
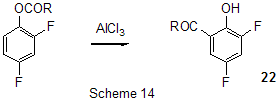
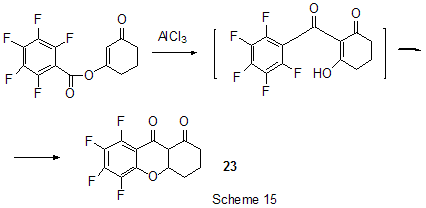
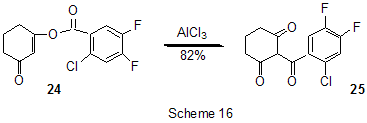
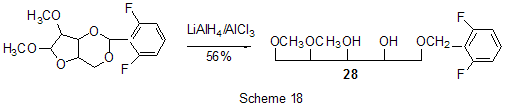
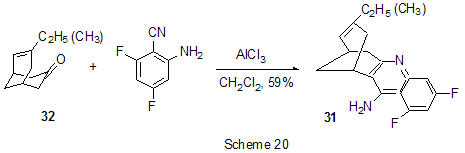
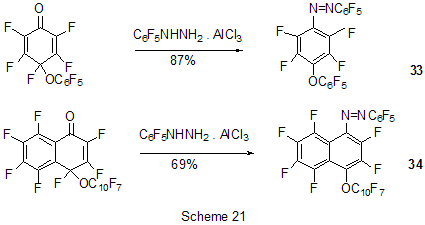
At last, acylation reactions with intramolecular cylization and without it were described for substrates containing a polyfluoroaromatic fragment when the acylation did not involve the polyfluorinated ring. The acylation of such type with cyclization was used in the multi-stage synthesis of potential inhibitors of cyclooxygenaze (COX-2), in particular in obtaining 2-(3',5'-difluorophenyl)-3-(4'-methylthiophenyl)cyclopenta-2-en-1-one (18) from 5-(3',5'-difluorophenyl)-4-(4'-methylthiophenyl)pentenic acid (19) [59] (Scheme 12), in producing 5-(2',4'-difluorobiphenyl-4-yl)-5-oxy-4-methyl-5H-furan-2-one in the acylation of 1,3-difluorobiphenyl with methylmaleic acid anhydride [44] and to form N-[6-(2,4-difluorophenoxy)-1-oxoindan-5-yl]methansulfonamide (20) from 3-[4-(2,4-difluorophenoxy)-3-methansulfonylaminophenyl]propionyl chloride (21) [60] (Scheme 13). The acylation without cyclization illustrates conversion of N-[3-(2,4-difluorophenoxy)-2-thienyl]methansulfonamide into N-[5-(3-chloro-1-oxopropyl)-3-(2,4-difluorophenoxy)-2-thienyl]methanesulfonamide [61] and conversion of 2,6-difluoro-4pyrrol-1-ylphenyl ether of benzoic acid into an appropriate compound with the benzoyl group at position 3 of the pyrrole ring [62]. The acylation reaction variant by Fries rearrangement was also realized, i.e. conversions of esters, phenols under the action of AlCl3 into ortho- and para-acylphenols. Thus, esters of 2,4-difluorophenol with acetic [63] or benzoic [64] acids are rearranged into stable (2'-oxy-3',5'-difluorophenyl)aceto- and -benzophenones 22 accordingly (Scheme 14) The rearrangement of 3.4-difluorophenylacetate results in 1-(4,5-difluoro-2-oxyphenyl)ethanone [65] and the rearrangement of 2,6-difluorophenyl ester of propionic acid results in (3,5-difluoro-4-oxyphenyl)propiophenone [66]. In contrast to that the reactions of 3-oxocyclohex-2enyl-2,6-difluoro-, -2,4-difluoro or 2,3,4,5,6-pentafluorobenzoates run with the formation of non stable products of the Fries rearrangement which are readily converted into appropriate derivatives of 2,3,4,9-tetrahydro-1H-xanten-1,9-dione 23 [67] (Scheme 15). Analogous cyclization runs also in case of 3-(2,4,5-trifluorobenzoyloxy)-2-cyclohexen-1-one, while appropriate 2-chloro-4,5-difluorophenylbenzoate (24) under the action of AlCl3 gives 2-(2chloro-4,5-difluorobenzoyl)-1,3-cyclohexandione (25)[68] (Scheme 16). 2. Dealkylation reaction (cleavage of the C-O bond) A known cleavage reaction of phenol ethers under the action of AlCl3 was used in obtaining 7-oxy-2-(3,4-difluorophenyl)-3-arylbenzo[b]thiophenes from 7-methoxy derivatives [16], 4-(4',5'-difluoro-2'-oxy-phenoxy) derivative of 2-substituted tetrahydropyran-3-ol from appropriate 2?-methoxy derivative [69] as well as in producing a 3-methoxyphenyl fragment of complex porphyrin compound with pentafluorophenyl groups in positions 10,20 [70]. Cleavage of O-C ether bond in cycles is achieved by reduction with LiAlH4/AlCl3 (1:2) system, as addition of AlCl3 weakens reductive ability of LiAlH4 in the system and thereby makes possible to avoid side reactions [71]. This procedure was used for producing (1R,2R)-1,2-diphenyl-2-(2,3,4,5,6-pentafluorobenzyloxy)ethanol (26) from (4R,5R)-α-(2,3,4,5,6-pentafluorophenyl)-4,5-diphenyl-1,3-dioxolane (27) [72] (Scheme 17) and for 1-(2,6-difluorobenzyloxy)-4,5-dimethoxypentan-2,3-diol (28) from 2-(2,6-difluorophenyl)-6,7-dimethoxytetrahydrofuro[3,2-d][1,3]dioxane [73] (Scheme 18). It should be noted that LiAlH4/AlCl3 system was also used for reduction of compounds of different types. In this was, for example, were obtained polyfluorophenyl ethylamines 29 from polyfluorophenyl acetonitriles 30 in 50-75% yield [74,75] (Scheme 19). 3. Condensations, isomerisations and reactions with nucleophiles New chlorohydrates of 12-amino-6,7,10,11-tetrahydro-7,11-methanocycloocta[b]quinolines (huprines) of 31 type containing a methyl or ethyl group as well as two fluorine atoms in positions 1,3 as potential inhibitors of acetylcholinesterase were synthesized by the interaction of bicycloenones 32 with 2-amino-4,6-difluorobenzonitrile according to the Friedländer reaction type with participation of carbonyl group [76](Scheme 20). The interaction of polyfluorinated 2,5-cyclohexadienes and 1-oxo-1,4-dihydronaphthalenes, containing pentafluorophenoxy- and perfluoronaphthoxy- fragments, with a complex of pentafluorophenylhydrazine and AlCl3 also involves the carbonyl group according to 1,2-addition reaction type in spite of the presence of fluorine atoms, sensitive to the action of nucleophilic agents, at the double bond in the cyclohexadienone fragment. The end products of these conversions as a result of aromatization are perfluoroaryloxy derivatives of nitrocompounds of benzene series 33 and naphthalene series 34 [77] (Scheme 21).
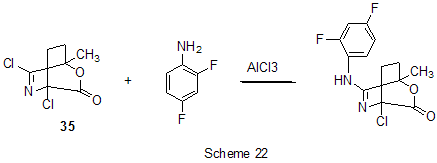
The absence of reactions involving fluorine atoms of the ring may be connected with reduced basicity of pentafluorophenylhydrazine including due to complexation with AlCl3. Reactions of nucleophilic agents in the presence of AlCl3 run smoothly enough if activation of the substrate is possible at the expense of formation of cationoid intermediates in the interaction of this substrate with AlCl3. Thus, activation of chloroimine function in 4,6-dichloro-1-methyl-2-oxa-5-azabicyclo[2,2,2]oct-5-en-3-one (35) by the action of AlCl3 allows realization of the reaction of this compound with 2,4-difluoroaniline [78] (Scheme 22).
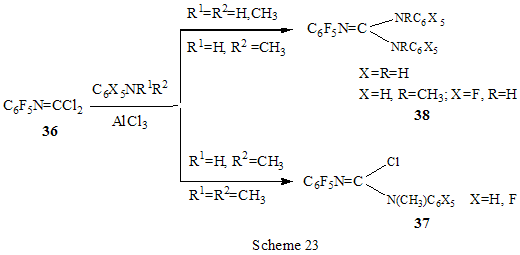
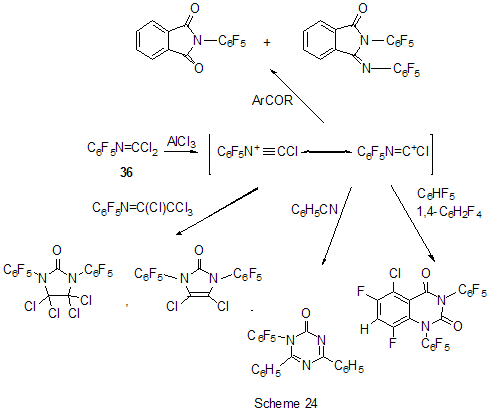
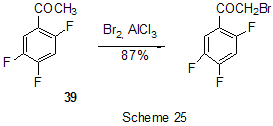
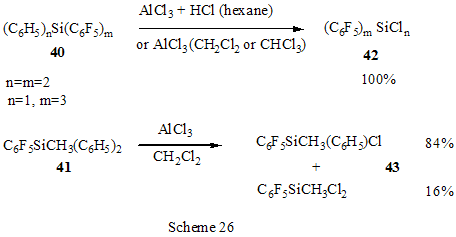
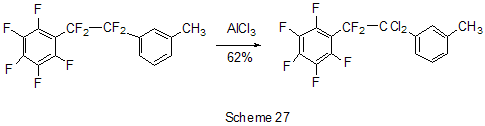
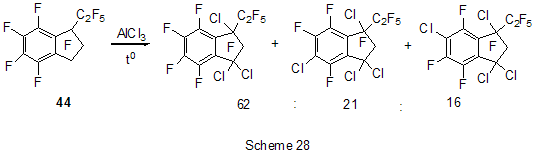
The reactions of polyfluoroarenes of such type, containing imidoylchloride group, run with polarization of C-Cl bond under the action of AlCl3 and formation of cationoid intermediate of nitrile- cation type. The interaction of positively charged intermediate with a nucleophile results in products of substitution of chlorine atoms for a nucleophile residue or in products of more complete conversions. For example, reactions of N-pentafluorophenylcarbonimidoyl dichloride (36) with aromatic amines ( aniline, N-methyl-, N.N-dimethylaniline and their pentafluorophenyl analogues) in the presence of AlCl3 and without it involve the amine nitrogen atom to form the same chloroformamidines 37 and guanidines 38 [79] (Scheme 23), but the conversion is higher in the reactions with aluminum chloride. It is explained by different mechanisms of the reactions in both cases. The reactions in the presence of AlCl3 run with participation of nitrile cation, whereas in the absence of AlCl3 a stage mechanism of bimolecular nucleophilic addition-detachment to form tetrahedral intermediate is realized [80,81].The reactions of dichloride 36 and AlCl3 with other arenes acting as the donor component ( compounds with multiple bond nitrogen-carbon [82], fluorinated benzenes [83], aromatic acids and their derivatives [84]) run with intramolecular heterocyclization. So, polyfluoroaromatic compounds containing -N=CClR group may be promising starting compounds for synthesis of fluorinated 5- and 6-membered N-heterocyclic systems (Scheme 24). Stereoselective isolation of trans-2-(3',4'-difluorophenyl)-6-propyldecahydronaphthalene from a mixture of isomers is realized by the mixture treatment with AlCl3 [85]. 4. Halogenation and hydrodefluorination reactions Conventionally AlCl3 has been used as a catalyst in halogenation reactions. In particular bromination of the methyl group of 2,4,5-trifluoroacetophenone (39) in the presence of AlCl3 is one of the stages in the multi-stage synthesis of 4-cyclopropyl-7-fluoro-6-(4-methylpiperazin-1-yl)-1,2,4,9-tetrahydrothiazol[5,4-b]quinolin-2,9-dione [86](Scheme 25). Catalysis by AlCl3 was also used for halogenation of the ring to produce halotetrafluorobenzenes C6F4XH (X=Cl, Br) in high yields from tetrafluorobenzenes [87]. In the presence of AlCl3 a phenyl group is replaced for fluorine in the synthesis of chloro- and chloro(methyl)pentafluorosilanes 42 and 43 from pentafluorophenyl(phenyl)silanes 40 and methyl(pentafluorophenyl)penylsilanes 41 accordingly [89,90] (Scheme 26). The reaction is of electrophilic character and proceeds under the action of AlCl3 itself or AlCl3/HCl system as well as another electrophiles. The conversion in these reactions depends on the solvent used (preferably halogenated hydrocarbon) and the number of electron-seeking pentafluorophenyl groups in silicon atom ( the greater the number the more difficult reaction course). At that Si-C6F5 and Si-Me bond is not cleaved under the action of AlCl3. AlCl3 may be used for substitution of fluorine atoms for chlorine in the benzyl position and in the aromatic ring of polyfluoroarenes containing fluoroaliphatic fragments. Thus, in 2-pentafluorophenyl-1-phenyltetrafluoroethane CF2 group was converted into CCl2 by the action of AlCl3 . The process of substitution of fluorine for chlorine runs only in the benzyl position of CH3C6H4CF2- fragment as a result of stabilization by the phenyl group of the forming positively charged center [90] (Scheme 27). In case of perfluoro-1-ethylindane (44) under the action of AlCl3 in dependence on its quantity and reaction temperature, both acyclic fluorine atoms of the 5-membered ring and aromatic fluorine atoms of the 6-membered ring are substituted for chlorine[91] (Scheme 28). The observed conversions are explained from point of view of possible formation of intermediate cations of different type and their reactivity. In case of 1,2,4,5-tetrafluoro-3-(2,2,2-trifluoro-1,1-bis-trifluoromethylethyl)-6-trifluoromethylbenzene under the action of AlCl3 fluorine atoms of the trimethyl group in position 6 are replaced for chlorine (85% yield) [92]. Recently it has been found that substitution of fluorine atoms by hydrogen is possible in polyfluorinated arenes , i.e. it is possible to realize hydrodefluorination process under certain conditions. Thus, regioselective hydrodefluorination of a trifluoromethyl group in perfluoroalkylbenzenes, for example in octafluorotoluene (48) into 1-methylpentafluorobenzene (49) is realized by heating with AlCl3 ,or with cyclic C5-6 compound( for example cyclohexane) or aliphatic C5-12 hydrocarbon in a medium of organochlorine solvent, for example CH2Cl2 , at 30-80oC [93]. 5. Another directions of AlCl3 using Complexes of fluorinated derivatives of tris-substituted borane compounds with Lewis bases may be destroyed by the action of strong Lewis acids, including AlCl3. In particular, adduct of tris(pentafluorophenyl)borane with TGF, derived from B(C6F5)3 and TGF after distillation of solvent excess under vacuum, is destroyed under the action of AlCl3 in 1,3,5-triisopropylbenzene at 25oC [94]. 6. Conclusion The analysis of publications presented in this report has shown that in spite of appearance of novel effective catalysts like Lewis acids in recent years, AlCl3 has not lost its importance as a catalyst and reagent in chemistry of polyfluoroarenes. The directions of its application are quite analogous to appropriate directions in non-fluorinated series and mainly come to the processes of Friedel-Crafts alkylation and acylation, that are most often realized as one of stages in complex multi-stage syntheses of promising biologically active substances or different practically important compounds. Also there are different variants of halogenation reactions with participation of AlCl3. An interesting direction of using AlCl3 appears polychloroalkylation of perfluorinated arenes with N-, O- and S-containing functional groups that unambiguously involves a heteroatom of the functional group, as a possibility of participation of the ring in the reaction is excluded, and results not only in primary products of polychloroalkylation but also in compounds of different types forming as a result of further conversions of these primary products. Activation of derivatives of polyfluorinated arenas by the action of AlCl3 to form cationoid intermediates is of certain interest as it makes possible to realize a reaction of such arenas with weak nucleophiles, whereas interaction of the latter under conventional conditions of aromatic nucleophilic substitution is rather difficult or does not occur at all. Thereby a scope of conversions with participation of weak nucleophiles is broaden and it makes possible to realize synthesis of compounds difficult to obtain in a different way. At last, the ability of AlCl3, earlier found, to reduce reductive properties of such strong reducing agent as LiAlH4 has been successfully employed in reactions of polyfluoroarenes, that made possible to decrease a probability of side processes and to obtain the desired products in good yields. References 1. F.A. Drahowzal, in: G.A. Olah (Ed.), Friedel-Crafts and Related Reactions, John Wiley, N.Y. etc., 1964, vol. 2, part 1. 2. G.A. Olah and W.S. Toloyesi, in: G.A. Olah (Ed.), Friedel-Crafts and Related Reactions, John Wiley, N.Y. etc., 1964, vol. 2, part 2. 3. T. Ooi, K. Maruoka, in: H. Yamomoto (Ed.), Lewis Acids in Organic Synthesis, VileyVCH Verlag GmbH, Weinheim-N.Y. etc., 2000, Vol.1, pp. 191-281. 4. M. Hudlicky, A.E. Pavlath, Chemistry of Organic Fluorine Compounds II, Am. Chem. Soc., Washington, DC, 1995. 5. R. Chambers, Fluorine in Organic Chemistry, Blackwell Publ., 2004. 6. M. Abid, M. Savolainen, S. Landge, J. Hu, C.K.S. Prakash, G.A. Olah, B. Török, J. Fluor. Chem., 128 (2007) 587-594. 7. X. Hao, A. Yoshida, J. Nishikido, J. Fluor. Chem., 127 (2006) 193-199. 8. C. G. Krespan, V.A. Petrov, Chem. Rev., 96 (1996) 3269-3302 9. Th. Krahl, E. Kemnitz, J. Fluor. Chem., 127 (2006) 663-678. 10. A. Dimitrov, D,Heidemann, E Kemnitz, Abstr. of 18th ISFC, Bremen, Germany, 2006, p. 80. 11. X. Hu, G.K. Chuah, S. Jaenicke, Appl. Catal. A, 209 (2001) 117-123; C.A. 134 (2001) 368543. 12. T. Koga, M. Yasutake, T.Slinmyozu, Org. Lett., 3 (2004)1419-1422. 13. R. Filler, A.E. Fiebig Jr., B.K. Mandal, J. Fluor. Chem., 102 (2000) 185-188. 14. M. Tabart, G. Picaut, J-F. Desconclois, S. Dutka-Malew, Y. Huet, N. Berthaud, Bioorg. and Med. Chem. Lett., 11 (2001) 919-922. 15. M. Acemoglu, T. Allmendinger, J. Calienni, J. Cercus, O. Loiseleur, G.H. Sedelmeier, Tetrahedron, 60 (2004) 11571-11586 . 16. G.V.P. Chandramouli, B. Prasanna, P.N. Kumar, P.V. Reddy, Phosphorous, Sulfur, and Silicon and Related Elements, 177 (2002) 511-522 . 17. T.D. Petrova, V.E. Platonov, Chemistry for Sustainable Development, 15 (2007) 511-523. 18. T.D. Petrova, V.E. Platonov, J. Fluor. Chem., 126 (2005) 860-876. 19. G.I. Borodkin, E.I. Chernyak, M.M. Shakirov, V.G. Shubin, Russ. J. Org. Chem., 34 (1998), 1634-1639. 20. V. Konovalov, S.S. Laev, I.V. Beregovaya, L.N. Shchegoleva, V.D. Shteingarts, Yu.D. Tsvetkov, I.I. Bilkis, J. Phys. Chem., A, 104 (2000) 352-361. 21. Zh. Li, N. Huang, Org. Prep. Proced. Int., 28 (1996) 245-248 . 22. J. Bartioli, E. Turmo, J. Belloc, j. Torn, J. Org. Chem., 60 (1995) 3000-3012 . 23. T. Kitazaki, A. Tasaka, H. Hosono, y. Matsushita, K. Itoh, Chem. Pharm. Bull., 47 (1999) 360-368. 24. D. Gala, D.J. DiBenedetto, I. Mergelsberg, M. Kugelman, Tetrahedron Lett., 37 (1996) 8117-8120. 25. A.K. Saksena, V.M. Girijavallabhan, H. Wang, Y-T. Liu, R.E. Pike, A.K. Canguly, Tetrahedron Lett., 37 (1996) 5657-5660. 26. K. Seno, T. Okuno, K. Nishi, Y. Murakami, K. Yamada, Sh. Nakamoto, T. Ono, Biorg. Med. Chem. Lett., 11 (2001) 587-590. 27. H. Kavanishi, H. Morimoto, T. Nakano, T. Watanabe, O.Kuniyuki, T. Tsajihara, Heterocycles, 49 (1998) 181-190. 28. Sh. Sonda, K. Katayama, T. Kawahara, W. Sato, K. Asano, Bioorg. Med. Chem., 12 (2004) 2737-2748. 29. R.G. Karki, V.M. Gokhall, P.S. Kharkar, V.M.Kulkarni, Ind. J. Chem., Sect.B, 42 (2003) 372-381. 30. M.L. Carmellino, G. Pagani, M. Pregholato, M. Terreni, V.Caprioli, F. Zani, Pestic Sci, 45 (1995) 227-236. 31. Sh. Ling, Zh. Xin, Zh. Qing, L. Jian-Bing, J. Zhong, F. Jian-Xin, Synth. Commun., 37 (2007) 199-207. 32. M.K. Bassapa, M.P. Sadashiva, K.S. rangappa, Ind. J. Chem., Sect.B, 43 (2004) 1954-1957. 33. J. Boivin, L. Ramos, S.Z. Zard, Tetrahedron Lett., 39 (1998) 6877-6880. 34. B. Bennetau, J. Mortier, J. Moyroud, J.L. Guesnet, Bull. Soc. Chim. Fr., 133 (1996) 133- 141. 35 M. Reuman, S.J. Daum, B. Singh, M.P.Wentland et al., J. Med. Chem., 38 (1995) 2531- 2540. 36. C. Bolm, A. Maischak, Synlett, 2001, 93-95. 37. A.P. Krapcho, M.J. Maresch, C.E. Gallagher, M.P. Hacker, J. Het. Chem., 32 (1995) 1693- 1702 . 38. H. Becker, K.B. Sharpless, Angew. Chem., 108 (1996) 447-449. 39. P. Szezecinski, Bull. Pol. Acad. Sci., Chemistry, 47 (1999) 25-32. 40. C.Y. Hang, S.H.Kim, K.Young, Bioorg. Med. Chem. Lett., 7 (1997) 1875-1878. 41. S. Jaime-Figueroa, L.J. Kurz, Y.Liu, R. Cruz, Spectrochim. Acta, A, 56 (2000) 1167-1178. 42. J.R. Tagat, S.W. McCombie, D.V. Nazareno, C.D. Boyle, J.A. Kozlowski, S. Chackalamannie, H. Josien, Yu. Wang, G. Zhou, J. Org. Chem., 67 (2002) 1171-1177. 43. J.J. Parlow, M.D. Mahoney, Pestic Sci, 46 (1996) 227-236. 44. M. Kuchar, Coll. Czech. Chem. Commun., 60 (1995) 1026-1033. 45. M. Artico, R.D. Santo, R. Costi, S. Massa, F. Scintu et al., Bioorg. Med. Chem. Lett., 7 (1997) 1931-1936 . 46. K.R. Romines, G.A. Freeman, L.T. Schaller, J.R. Cowan et al., J. Med. Chem., 49 (2006) 727-739. 47. F. Caturia, J-M. Jimenez, N. Godessart, M. Amat, A. Cardenas, L. Soca, J. Beleta, H. Ryder, M.I. Crespo, J. Med. Chem., 47 (2004) 3874-3886. 48. H. Maeda, A. Osuka, Y. Tshikawa, I. Aritome, Y.Hisaeda, H. Furuta, Org. Lett., 5 (2003) 1293-1296 . 49. J.A. Letizia, A. Facohetti, Ch.I. Stern, M.A. Rathner, T.J. Marks, J. Amer. Chem. Soc., 127 (2005) 13476-13477 . 50. M.G. Ryadnov, N.YA. Kashparova, I.A. Kashparov, Yu.V. Mitin, Bioorg. khimiya, 24 (1998) 408-411. 51. M.C. Cardia, M. Begala, A. Delogu, E. Maccioni, Farmaco, 56 (2001) 549-554. 52. N. Martin, P. de Miguel, C. Seoane, A. Albert, F.H. Cano, J. Mater. Chem., 7 (1997) 25-30. 53. Y. Sakamoto, T. Suzuki, M. Kobayashi, y. Gao, Y. Fukai, Y. Inoue, F. Sato, S. Tokito, J. Amer. Chem. Soc., 126 (2004) 8138-8140. 54. M. Kobayashi, O. Omae, K. Ohkubo, G. Kimitake, Jap. Pat. Intern. Appl. WO 2005154337A2 [C.A. 142 (2005) 463466]. 55. T. Kasumoto, Y. Sato, Y. Nagashima, M. Negishi, S. Takehara, Y. Iwashita, K. Takeuchi, H. Takatsu, C. Pithart, R. Frings, A. Lachowicz, G. Grahe, Molecular Crystal and Liquid Crystals, 411 (2004) 155-162) [C.A. 142 (2005) 325812k]. 56. M. Sugimori, A. Ejima, S. Ohsuki, K. Uoto, I. Mitsui, Y. Kawato, y. Hirota, K. Sato, H. Terasawa, J. Med. Chem., 41 (1998) 2308-2318. 57. D.L Musso, F.R. Cochran, J.L Kelley, E.W. McLean, J.L. Selph, G.C. Rigdon et al., J.Med. Chem., 46 (2003) 399-408. 58. H-J. Li, G.A. Boswell, Tetrahedron Lett., 37 (1996) 1551-1554 . 59. D. Zhao, F. Xu, Ch. Chen, R.D. Tillyer, E.J.J. Gralowski, P.J. Reider, Tetrahedron, 55 (1999) 6001-6018. 60. Ch. Li, C. Soncy-Breau, N. Quimet, Synthesis, 1995, 1355-1356. 61. D. Binder, M. Pyerin, R. Steindl, M.Weisgram, Monatshefte Chem., 129 (1998) 887-896. 62. I. Nicolaou, C. Zika, V.J. Demopoulos, J. Med. Chem., 47 (2004) 2706-2709. 63. J.K. Lynch, J.C. Freeman, A.S. Yudd, R. Iyengar, M. Mulchern, G. Zhao, J.J. Napier et al., J. Med. Chem., 49 (2006) 6569-6584. 64. M. Krause, A. Ronleau, H. Stark, M. Garbarg, J.C. Schartz. W. Schunaek Pharmazie, 51 (1996) 720-726. 65. D.A. Vasselin, A.D. Westwell, Ch.S. Mathews, T.D. Bradshaw, M.F.G. Stevens, J. Med. Chem., 49 (2006) 3973-3981 . 66. D. Musso, J.L. Killey, Tetrahedron Assimetry, 6 (1995) 1841-1844. 67. Ch.D. Gabbutt, J.D. Hepworth, M.W.J. Urquhart, L. Millan Vazguez de Miguel, J. Chem. Soc., P I, 1998, 1819-1824. 68. S Radl, Collect. Czech. Chem. Commun., 60 (1995) 2127-2136. 69. Sh. Masumoto, K. Yabu, M. Kanai, M. Shibasaki, Tetrahedron Lett., 43 (2002) 2919-2922. 70. H-A. Wagenknecht, C. Claude, W.D. Woggon, Helv. Chim. Acta, 81 (1998) 1506-1520. 71. R.F. Nystrom, J. Amer. Chem. Soc., 77 (1955) 2544-2545. 72. P. Scafarо, L. Leo, S. Soperchi, C. Rosini, Tetrahedron, 58 (2002) 153-160. 73. Chig-yun Hsu, Chia-Chun Chen, Synth. Commun., 33 (2003) 2349-2363. 74. R. Filler, W. Chen, S.M. Woods, J. Fluor. Chem., 73 (1995) 95-100. 75. B.K. Das, N. Shibata, Y. Takeuchi, J. Chem. Soc., P 1, 2002, 197-206. 76. P. Camps, R. ElAhab, J. Morral, D. Munoz-Torreto, A.Badia, J.E.Banos, N.M. Vivas, X. Barril, M. Orozco, F.J. Luque, J. Med. Chem., 43 (2000) 4657-4666. 77. V.N. Kovtonyuk, L.S. Kobrina, Russ. Chem. Bull., 45 (1996), 1688-1691. 78. K.J. Dubois, Ch.C. Fannes, S.M. Toppet, G.J. Hoornaert, Tetrahedron, 52 (1996) 12529- 12540. 79. T.D. Petrova, V.E. Platonov, Russ. J. Org. Chem., 33 (1997) 684-792. 80. V.A. Mihajlov, I.V. Kolesnikova, F.F. Popov, T.D. Petrova, V.E. Platonov, V.A. Savelova, J. Org.Chem. USSR, 25 (1989) 927- 932. 81. V.A. Mihajlov, A.A. Popov, V.F. Savelova, I.V. Kolesnikova, T.D. Petrova, V.E. Platonov, J. Org. Chem. USSR, 25 (1989) 1517- 1522. 82. T.D. Petrova, V.E. Platonov, I.V. Kolesnikova, T.V. Ribalova, I.Yu. Bagryanskaya, Yu.V. Gatilov, J. Fluor. Chem., 103 (2000) 63-73. 83. T.D. Petrova, V.E. Platonov, L.M. Pokrovskii, T.V. Ribalova, Yu.V. Gatilov, Collect. Czech. Chem. Commun., 67 (2002) 1449-1466 . 84. T.D. Petrova, V.E. Platonov, Russ. J. Org. Chem., 41 (2005). 85. A. Kusumoto, S. Takehara, Y. Akachi, JP 2003026618 A2, (2003) [C.A. 138 (2003) 122467 ]. 86. S. Radl, Collect. Czech. Chem. Commun., 62 (1997) 791-799 . 87. G. Borsotti, A. Sommazi, R. Santi, Ital. PCT Int. Appl. WO 2000076946 A1 (2000) [C.A. 134 (2001) 41969 ]. 88. H-J. Frohn, A. Lewin, V.V. Bardin, J. Organomet. Chem., 568 (1998) 233-240. 89. H-J. Frohn, A. Lewin, V.V. Bardin, J. Organomet. Chem., 570 (1998) 255-263. 90. Zh. Yu Yang, D.J. Burton, J. Fluor. Chem., 102 (2000) 89-103. 91. M.S. Vasilevskij, V.M. Karpov, T.V. Mezhenkova, V.E. Platonov, Russ. J. Org. Chem., 35 (1999). 92. V.I. Krasnov, V.E. Platonov, Russ. J. Org. Chem., 36 (2000) 1488-1498. 93. S.M. Igumnov, A.M. Krokhalev, R.Kh. Nefedova, RU 2182570 C2 (2002) [C.A. 138 (2003) 385159]. 94. Anon (UK). Res. Discl. 397 (May), P 355, N 39779 (1997) [C.A. 127 (1997) 5116]. |
Fluorine Notes, 2009, 63, 3-4