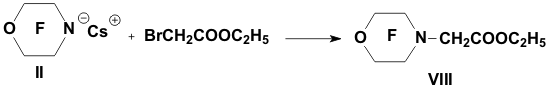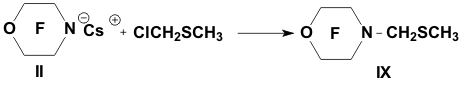Fluorine Notes, 2009, 63, 1-2
REACTIVITY OF N-PERFLUOROMORPHOLYNYL AZA-ANIONGrinevskaja V.K., Del'tsova D.P., Gervits L.L. A.N. Nesmeyanov Institute of Organoelement Compounds, the Russian
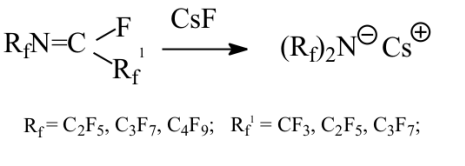
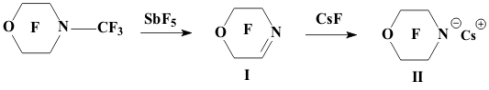
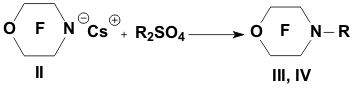
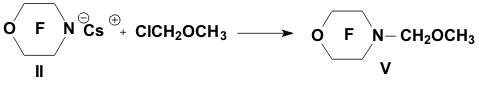
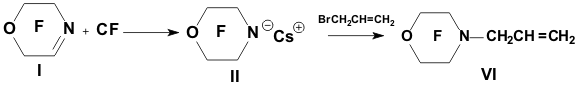
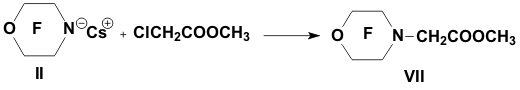
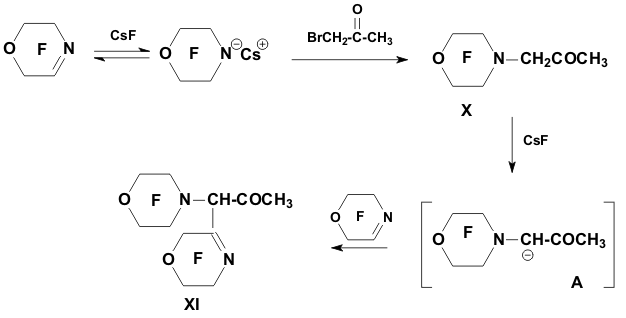
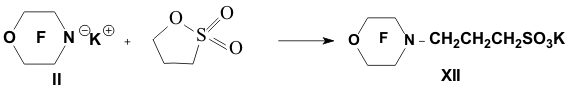
Academy of Sciences. Vavilova St.,28, 119991, Moscow, Russian Federation Previously generation of bis(trifluoromethyl)aza anion from perfluoro-2-azapropene and CsF (KF) has been described [1,2]. The reactions of this anion were studied extensively and it has been shown that they provide a convenient method for the synthesis of various compounds containing the bis(trifluoromethyl)amino group. Later it was found that other perfluoroazaalkenes react with alkali metal fluorides in aprotic polar solvents to form stable aza-anions [3]. Recently perfluoro-N-methylmorpholine has become a rather available compound. The reaction of perfluoro-N-methylmorpholine with SbF5 leads to perfluoromorpholinimine (I) in good yield. It turned out that perfluoromorpholinimine (I) also reacts with alkali metal fluorides in aprotic solvents to give the corresponding stable perfluorinated aza-anion (II) (according to 19F NMR data). We have studied the reactivity of aza-anion (II) in its reactions with various alkylating agents. Aza-anion (II) reacts with dimethyl- and diethylsulfate to give the corresponding N-alkylsubstituted perfluoromorpholines (III and IV). R = CH3, C2H5 The reaction of anion (II) with methyl chloromethyl ether also occurs smoothly to give N-methoxymethylperfluoromorpholine (V) in good yield. Perfluoromorpholynyl N-anion (II) readily reacts with allyl bromide to form N-allylperfluoromorpholine (VI) in high yield (73%). Reaction of aza-anion (II) with methyl chloroacetate proceeds slowly even when heated (40 to 50oC) to give the expected product (VII) in low yield (28%). Alkylation of aza-anion II with ethyl bromoacetate occurs much faster than in the case of methyl chloroacetate. In accordance with these data we have developed a procedure for the synthesis of ethyl perfluoromorpholinoacetate (VIII). Aza- anion II is easily alkylated with chloromethyl methyl sulfide in diglyme to give product (IX) in 65% yield. When aza-anion II reacts with bromoacetone, the process does not stop at the step of formation of the alkylation product (X). Under the reaction conditions the mobile hydrogen atom at α-position to the carbonyl group is eliminated, and the intermediate carbanion A attacks the starting aza-alkene I to give product (XI) as a result of nucleophilic substitution of the fluorine atom. When the anion II reacts with propane sultone, the ring opening readily occurs at room temperature to give 3-(N-perfluoromorpholynyl)propane sulfonic acid potassium salt (XII). The reaction proceeds smoothly if diglyme is used as a solvent. In the case of acetonitrile the reaction is more prolonged, but the yield is also good (96%).
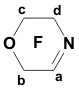
The data on the reactivity of perfluoromorpholynyl aza-anion obtained in this study allow us to consider it as a useful reagent for introducing the fluorine-containing moiety into various organic molecules. EXPERIMENTAL 19F NMR spectra and 1H NMR spectra were recorded on a Bruker AC-200 X instrument (200 MHz for 1H and 188.3 MHz for 19F NMR, respectively) with TMS and CF3COOH used as references, respectively. Chemical shifts are given in ppm using (1H) and (19F) scales, the coupling constants reported in Hz. Mass-spectra were obtained on a VG 7070E spectrometer (ionizing electron energy 70 eV). The course of the reactions was monitored by 19F NMR spectroscopy. Synthesis of perfluoromorpholinimine (I) Perfluoro-N-methylmorpholine (22.5 g, 0.07 mol) and SbF5 (4.34 g, 0.02 mol) were placed in a steel autoclave and heated at 100oC for 4 h. Then 12.4 g of product (I) (bp 22-24oC) was distilled off from the reaction mixture. Yield 84%. 19F NMR (δ, ppm): -21.3 (Fa), 0.3 (2F b), 14.6 (2F c), 20.4 (2F d); Ja-d= 8 Hz; Ja-b = 22.0 Hz; Jb-c = 6 Hz. Mass spectra (m/z, intensity, %): 192 [M-F]+(5); 164 [M-COF]+ (16); 145 [M-COF2]+ (78); 142 [M-CF3]+ (9); 119 [C2F5]+ (21); 114 [C2F4N]+ (22); 100 [C2F4]+ (100); 95 [C2F3N]+ (43); 92 [M-C2F5]+ (17); 69 [CF3]+ (100). Synthesis of N-methylperfluoromorpholine (III) Dry CsF (3.0 g, 0.019 mol) and dry diglyme (20 ml) were placed in a flask fitted with a reflux condenser cooled with solid CO2. Then perfluoromorpholinimine (I) (4.2 g, 0.019 mol) was added dropwise. The reaction mixture was stirred for 30 min and (CH3)2SO4 (2.4 g, 0.019 mol) was added dropwise with stirring. After completing the addition, the reaction mixture was stirred at room temperature for 2 h. A fraction with bp .68-74oC was distilled off from the reaction mixture and redistilled to give 3.8 g of N-methylperfluoromorpholine III (bp 70-72oC). Yield 82%. 19F NMR (δ, ppm): 9.5 (CF2NCF2); 21.6 (CF2OCF2). 1H NMR (δ, ppm): 3.29 (CH3).Mass spectra (m/z, intensity, %): 245[M]+ (20); 226 [M-F]+(9);179 [M-CF2O]+ (10); 176 [M-CF3]+ (19); 100 [C2F4]+ (100); 69 [CF3]+ (15); 60 [CFNCH3]+ (10); 15 [CH3]+ (7). Synthesis of N-ethylperfluoromorpholine(IV) Dry CsF (3.5 g, 0.023 mol) and dry diglyme (20 ml) were placed in a flask fitted with a reflux condenser cooled with solid CO2. Then perfluoromorpholinimine (I) (4.86 g, 0.023 mol) was added dropwise. The reaction mixture was stirred for 50 min and (C2H5)2SO4 (4.62 g, 0.023 mol) was added dropwise with stirring. After completing the addition, the reaction mixture was stirred at room temperature for 12 h. A fraction with bp.78-140oC was distilled off from the reaction mixture and redistilled to give 3.7 g, of N-ethylperfluoromorpholine IV (bp 89-90oC). Yield 63%. 19F NMR (δ, ppm): 10.8 (CF2NCF2); 20.2 (CF2OCF2).1H NMR (δ, ppm): 1.58 (CH3); 3.68 (CH2); J = 5Hz. Mass spectra (m/z, intensity, %): 259 [M]+ (5); 244 [M-CH3]+(100); 240 [M-F]+(2); 212 [M-COF]+ (12); 119 [C2F5]+ (20); 100 [C2F4]+ (20); 69 [CF3]+ (10); 29 [C2H5]+ (74). Synthesis of N-(methoxymethyl)perfluoromorpholine (V) Dry CsF (7.2 g, 0.047 mol) and dry diglyme (50 ml) were placed in a flask fitted with a reflux condenser cooled with solid CO2. Then perfluoromorpholinimine (I) (10.0 g, 0.047 mol) was added dropwise. The reaction mixture was stirred for 30 min, and ClCH2OCH3 (3.78 g, 0.047 mol) was added dropwise with stirring. After completing the addition, the reaction mixture was stirred at room temperature for 2 h. A fraction with bp.60-140oC was distilled off from the reaction mixture and redistilled to give 10.2 g of N-(methoxymethyl)perfluoromorpholine V (bp 106-108oC). Yield 79%. 19F NMR (δ, ppm): 11.2 (CF2NCF2); 18.5 (CF2OCF2).1H NMR (δ, ppm): 3.67 (CH3); 5.15 (CH2). Mass spectra (m/z, intensity, %): 274 [M-H]+(2.3); 256 [M-F]+(1.8); 244 [M-OCH3]+ (42); 194 [M-OCH3-CF2]+ (42); 119 [C2F5]+ (2.4); 100 [C2F4]+ (14); 45 [CH2=OCH3]+ (100). Synthesis of N-allylperfluoromorpholine (VI) Perfluoromorpholinimine I (21.1 g, 0.1 mol) was added dropwise with vigorous stirring to a mixture of cesium fluoride (15.2 g, 0.1 mol) and 80 ml of dry diglyme introduced in a flask fitted with a thermometer, a dropping funnel, and a reflux condenser cooled with solid CO2. After vigorous stirring for 30 min, allyl bromide (12.1 g, 0.1 mol) was added dropwise to the reaction mixture and the mixture was stirred for 8 h at room temperature (formation of CsBr precipitate was observed). A fraction with bp.80-140oC was distilled off from the reaction mixture and redistilled to give 20 g of N-allylperfluoromorpholine VI (bp 101-102oC), of 96% purity (GLC). Yield 73.8%. 19F NMR (δ, ppm): 11.5 (CF2NCF2); 20.7 (CF2OCF2).1H NMR (δ, ppm): ABCX2-system: 4.2 d (-NCH2), J (CH2-CH )= 5.9 Hz; 5.55 d (=CHB), J (HB- CH) = 10.5 Hz; 5.66 d(=CHA), J (HA- CH) = 17.5 Hz; 6.2 ddt (CH), J (CH - CHA) = 17.5 Hz, J (CH - CHB)= 10.5 Hz, J(CH-CH2)=5.9 Hz. Mass spectra (m/z, intensity, %): 271[M]+(45); 244 [M-C2H3]+(10);164 [C3F6N]+ (9); Synthesis of methyl( perfluoromorpholynyl)acetate (VII) A solution of perfluoromorpholynyl aza-anion (II) prepared by the common procedure from 12.5 g (0.059 mol) of perfluoromorpholinimine (I), 9 g (0.059 mol) of CsF, and 80 ml of acetonitrile. To the solution obtained, methyl chloroacetate (6.4 g, 0.059 mol) was added dropwise with stirring. The reaction mixture was heated at 40-50?C with vigorous stirring for 30 h. Then the reaction mixture was filtered, acetonitrile was distilled off. Distillation of the residue gave 5 g (28%) of ester (VII), bp 152-155oC. 19F NMR(δ, ppm): 11.0 (CF2OCF2); 19.8 (CF2NCF2).1H NMR(δ, ppm): 3.9 (OCH3); 4.3 (CH2). Mass spectra (m/z, intensity, %): 303 [M]+( 2); 256 [M-F-CO]+(14); 244 [M-CH3CO2,]+ (66); 59 [CH3COO]+(100); 15 [CH3]+( 28). Synthesis of ethyl( perfluoromorpholynyl)acetate (VIII) To a mixture of dry CsF (25.9 g, 0.17 mol) and 200 ml of diglyme in a flask fitted with a reflux condenser cooled with solid CO2, perfluoromorpholinimine (I) (36.0 g, 0.17 mol) was added with stirring. The reaction mixture was stirred for 30 min. Ethyl bromoacetate (28.5 g, 0.17 mol) was added dropwise, and the reaction mixture was stirred at room temperature for 18 h. Then the reaction mixture was poured into water, the lower layer was separated, washed with water, dried over MgSO4, and distilled. Distillation gave 36 g of product VIII, bp 87oC/65 mm. Yield 67%. 19F NMR (δ, ppm): 10.0 (CF2OCF2), 19.0 (CF2NCF2).1H NMR(δ, ppm): 1.36 (t, CH3), 3.2 (CH2-N), 3.3 (q, CH2). Mass spectra (m/z, intensity, %): 244 [M - COOC2H5]+ (90); 119[C2F5]+ (10); 100 [C2F4]+ (13); 29 [C2H5]+ (100). Synthesis of N-(methylthiomethyl)perfluoromorpholine (IX) Dry CsF (20.0 g, 0.132 mol) and dry diglyme (180 ml) were placed in a flask fitted with a reflux condenser cooled with solid CO2. Then perfluoromorpholinimine (I) (27.8 g, 0.132 mol) was added dropwise. The reaction mixture was stirred for 50 min, and ClCH2SCH3 (12.7 g, 0.132 mol) was added dropwise with stirring and the reaction mixture was stirred when heated at (35-40oC for 1.5 h, then at room temperature for 18 h. After completing the process, the reaction mixture was poured into water, the lower layer was separated and dried over MgSO4. Distillation gave 25 g of product IX, bp 142-144oC. Yield 65%. 19F NMR(δ, ppm): 11.4 (CF2OCF2), 20.1 (CF2NCF2).1H NMR (δ, ppm): 2.4 (CH3); 4.7 (CH2). Mass spectra (m/z, intensity, %): 291 [M]+ (40); 244 [M-SCH3]+(100); 119 [C2F5]+( 30); 78 [CF2CO]+(51). Reaction of perfluoromorpholinimine with bromoacetone Dry CsF (6 g, 0.039 mol) and dry diglyme (80 ml) were placed in a flask fitted with a reflux condenser cooled with solid CO2. Then perfluoromorpholinimine (I) (8.4 g, 0.039 mol) was added dropwise with stirring. After completing the addition, the reaction mixture was stirred at room temperature for 30 min and cooled to 0oC. Then bromoacetone (5.5 g, 0.039 mol) was added dropwise. After completing the addition, the reaction mixture was stirred at room temperature for 30 min and poured into water; the lower layer is separated and dried over MgSO4. According to 19F NMR and Mass spectra data the crude product contained perfluoromorpholinoacetone X and 1-perfluoromorpholino-1-(2'-perfluoromorpholinimino) acetone (XI) in a 1:1 ratio. (X): 19F NMR(δ, ppm): 11.2 (CF2OCF2), 20.5 (CF2NCF2).1H NMR (δ, ppm): 2.4 (CH3); 4.7 (CH2). Mass spectra (m/z, intensity, %): 268 [M-F]+(5);244 [M-COCH3]+ (12); 100 [C2F4]+(15); 78[C2F2O]+ (17); 57 [CH2COCH3]+ (45); 43 [COCH3]+ (100); 15 [CH3]+ (50). (XI): 19F NMR(δ, ppm): -1.95 (OCF2C=), 11.9 (CF2OCF2), 14.7 (OCF2), 18.5 (CF2N), 21.8 (CF2NCF2). 1H NMR (δ, ppm): 2.39 (CH3); 6.38 (CH). Mass spectra (m/z, intensity, %): 477 [M-H]+ (1); 459 [M-F]+(2); 248[M-C2F4OC2F2N]+ (100); 119[C2F5]+ (19); 100 [C2F4]+ (42); 78 [C2F2O]+ (10); 43 [COCH3]+ (21). Synthesis of 3-(perfluoromorpholynyl)propanesulfonic acid potassium salt (XII) Perfluoromorpholinoimine (I) (17.5 g, 0.083 mol) was added dropwise with stirring to a mixture of KF (spray dried) (4.8 g, 0.083 mol) and 150 ml of dry acetonitrile placed in a flask fitted with a thermometer, a dropping funnel, and a reflux condenser cooled with solid CO2. The reaction mixture was kept for 12 h, and a solution of propane sultone (10.1 g, 0.083 mol) in dry acetonitrile (30 ml) was added. The mixture obtained was stirred for 8 h and filtered. The precipitate was washed with diethyl ether and dried. Salt XII (31 g, 96%) was obtained. 19F NMR (DMF-D8): 9.0 (CF2OCF2), 17.9 (CF2NCF2). 1H NMR: 2.1 (t, CH2); 2.7 (t, CH2S); 3.8 (t, J=7.4 Hz, CH2N); Anal. Calcd for C7F8H6NSO4K : C, 21.48; H, 1.53; F, 38.87; N, 3.58%. Found: C, 20.28; H, 1.66; F, 37.32; N, 3.58%. REFERENCES 1. A.F. Gontar, E.G. Bykhovskaya, I.L. Knunyants. Zh. Vses. Khim. O-va, 20, 232 (1975) 2. A.F. Gontar, E.G. Bykhovskaya, I.L. Knunyants. Izv.Akad.Nauk SSSR, Ser. Khim. 1975, 212 3. V.K. Grinevskaya, V.A. Petrov, M.A. Kurikin and L.S. German, J of Fluorine Chem., v.58, N 2-3, 176 (1992) |
Fluorine Notes, 2009, 63, 1-2