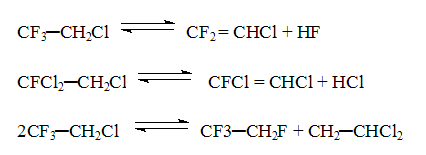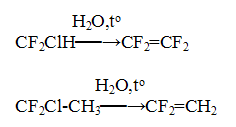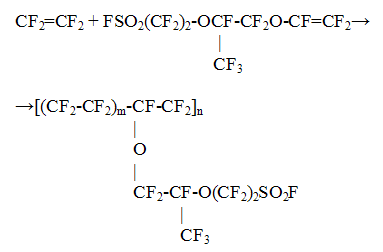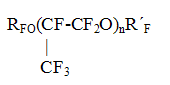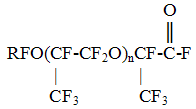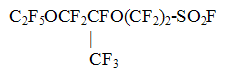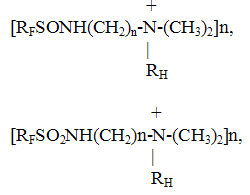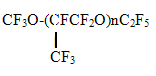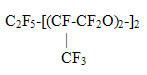Fluorine Notes, 2009, 62, 3-4
Commercial fluorine containing compoundsV.G.Barabanov, B.N.Maximov FSUE RSC "Applied Chemistry" Saint Petersburg, Russia The engineering studies in the field of commercial fluorine containing compounds are given for the following: ozone-friendly hydrofluorocarbons ( chladones), fluoroolefines, compounds containing functional groups, heat-resistant liquids, oils, lubricants, fluorine surfactants etc.. 1. Ozone-friendly compounds. Chlorofluorocarbons and bromofluorocarbons (chladones and halons) as compounds possessing a number of unique properties such as chemical inertness, non-toxicity, fire and explosion safety, were widely used in engineering as aerosol propellants, refrigerants, foaming agents for plastics, solvents, highly efficient extinguishers etc. But a destructive impact of some chlorine- and bromine-containing compounds on the ozone layer discovered in the 1980 drew attention to a large group of commercial chladones containing chlorine and bromine in their composition because of their potential danger. Up-to-date evaluations of relative role of antropogenic and natural factors of the ozone layer depletion have shown that the relative contribution of the antropogenic factors makes 75% over Western Europe, 60% over Eastern coast of the USA, 50% over Japan, Baikal and the Far East. The Vienna Convention for the protection of the ozone layer signed in 1985y was the first international document envisaging measures for the protection of the ozone layer. The countries, that signed the Convention, undertook obligations on collaboration in the field of control and prevention of the activity potentially dangerous to the stratosphere ozone layer. In September 1987 the Montreal Protocol (MP) was passed on the substances depleting the ozone layer, with the Annex listing ozone depleting substances (ODS) subject to the control by all the countries parties to the MP. It contained chlorofluorocarbons (CFC), bromofluorocarbons (halons) and some chlorohydrocarbons( CHC). The Protocol laid the obligations on the Parties to limit consumption, production and import/export of ODS. The Soviet Union was one of the first-rate producers of ODS: in 1987 its share in production of ODS (~200thous.tons/year) was around 15% of the world production, at the same time the Soviet Union was one of the main exporters of ODS supplying not only all republics of the former USSR but also a number of countries of Europe and Asia. Almost all industrial branches consumed chladones in Russian Federation [1,2.3]. The structure of consumption in 1990y was the following: 27% for production, repair and service of domestic and commercial industrial refrigerating equipment, 45% for production of goods in aerosol packages, 11% for production of foam materials, 14% for the use of chladones as solvents, 2% for fire extinguishing, 1 % for other purposes. The Russian Federation, being the assignee of the USSR, ratified the Montreal Protocol on ODS accepted all the obligation on the Protocol to terminate production and consumption of ODS. RSC "Applied Chemistry" together with a number of institutes of the country has fulfilled a great work volume to transfer the industry to the new class of chemical compounds instead of the prohibited ODS. As a result of the fulfilled investigations the following nomenclature of the new chladones was proposed: hydrofluorocarbons 134a(CF3CFH2), 152a(CF2HCH3), 125(CF3CF2H), 32(CH2F2) etc. The main difference of ozone-friendly substances from ODS was in the absence in their molecules of chlorine and bromine atoms which may participate in "chlorine" and "bromine" cycles of the ozone depletion. Beside the zero value of ODP ( ozone depleting potential) and an acceptable value of GWP ( global warming potential) the main criteria in the choice of the alternatives of ODS was the proximity of physical and chemical and operational properties to the analogues characteristics of ODS to be replaced. The basic nomenclature of ozone-friendly compounds, accepted for development and implementation in commercial production in the Russian Federation is given in Table 1. Table 1. MAIN CHARACTERISTICS OF HYDROFLUOROCARBONS (OZONE-FRIENDLY CHLADONES)
The development of technologies for ozone-friendly fluorohydrocarbons in dependence on their structure and taken into account the existing production capacities of CFC 11, 12, 113 to be replaced was realized at the plants of Russian Federation (RF) by main two production methods: liquid phase fluorination with anhydrous hydrogen fluoride of chloroorganic compounds (HFC 52a, 32, 143a and etc.) and gas phase catalytic fluorination of chloroorganic compounds with anhydrous hydrogen fluoride (chladones 134a, 125 and etc.) The main materials for manufacture of ozone friendly chladones ( trichloroethylene, perchloroethylene, vinylidene chloride, vinyl chloride and chloromethanes) are produced at the enterprises of RF : JSC "Caustic",Volgograd, JSC "Khimprom", Volgograd, JSC "Caustic" ,Sterlitamak, JSC "Usolyekhimprom" , Usolye Sibirskoe and etc. Special features of the liquid phase catalytic hydrofluorination are seen on the example of difluoromethane (chladone 32) synthesis [4]. The synthesis process proceeds according to scheme 1 Scheme 1 The kinetics of methylene chloride fluorination is described by the following equations [5] : where CCH2Cl2, CCH2ClF, Ck are the concentrations of dichloromethane, chlorofluoromethane and catalyst accordingly. The kinetics data have been used as a basis for calculation of a reactor for synthesis of CFC-32 of annual production capacity of 1000 tons and for development of a flow chart of the synthesis of CFC-32 which is typical for liquid-phase synthesis of ozone-friendly substances. The synthesis is carried out at a temperature of 95-105 oC at pressure of 1.5-2.0 MPa. The synthesis of CFC 152a from vinyl chloride and hydrogen fluoride proceeds according to the similar scheme at a temperature of 90oC and pressure of 0.6-0.8 MPa in the presence of the catalyst of tin tetrachloride [6], as well as the synthesis of CFC 245fa from pentachloropropane and hydrogen fluoride in the presence of SbCl5 at a temperature of 100-120oC and pressure of 1.6-1.8 MPa [7]. But the liquid-phase hydrofluorination method has a number of the limitations: - it is quite difficult to replace the chlorine atom for the fluorine atom in -CH2Cl
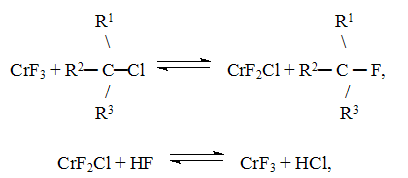
and =CHCl groups , Therefore in order to obtain hydrofluorocarbons with a substantial fluorine content in the molecule (CF3CFH2, CF3CF2H) the method of gas phase catalytic hydrofluorination is used. The peculiar features of the gas phase catalytic hydrofluorination are well seen from the example of synthesis of 1,1,1,2-tetrafluoroethane ( CFC 134a) from trichloroethylene and hydrogen fluoride. The synthesis process runs in two stages (scheme 2). Scheme 2 C2HCl3 +3HF C2H2ClF3 + HF As it follows from the experimental data and thermodynamical calculation of stage (1) the content of CFC 133a in organic part of the synthesis products may reach 90-98%. Stage (2) is reversible. In dependence on the conditions the content of CFC134a in organic part of the synthesis products may attain 10-40% (of organic part of the synthesis products). A chromomagnesium fluoride catalyst used in the process was made by impregnation of a magnesium fluoride powder with a solution of chromium chloride with subsequent mixing, molding, drying and treatment with hydrogen fluoride to transfer CrCl3 to fluoride CrF3 [9,10]. The investigation of the mechanism of the catalyst action in this process has sown that the catalyst acts as a carrier of fluorine and chlorine atoms [11,12]. It may be considered that the process of hydrofluorination of fluoroethanes proceeds according to scheme 3. Scheme 3 where chromium fluoride replaces chlorine by fluorine in fluorochloroethanes by changing its composition to CrF2Cl and after that hydrogen fluoride replaces chlorine by fluorine in active part of the catalyst to form hydrogen chloride and CrF3. Besides main hydrofluorination processes the reactions of dehydrofluorination, dehydrochlorination and disproportionation are carried out in the presence of this catalyst (scheme 5). Scheme 4 The obtained unsaturated fluorine-containing compounds( especially CF2=CHCl forming the azeotrope with 1,1,1,2-tetrafluoroethane) subsequently make difficult isolation of end product from the reaction mixture. The mechanism of the reaction of trichloroethylene hydrofluorination has been studied and laid as a basis for a kinetic model of the synthesis of 1,1,1,2-tetrafluoroethane [14] with the use of route method for complex reactions [13]. A typical flow chart for the synthesis of ozone-friendly substances was developed by a method of gas-phase catalytic hydrofluorination. In the Russian Federation have been created and continue to be created industrial facilities for production of ozone-friendly substances on the basis of the conducted investigations. Table 2. Production of ozone-friendly CFCs at the plants in RF
KCCW- Kirovo-Chepetsk Chemical Works, OJSC, A new class of ozone-friendly substances, fluorine-containing ethers, given in Table 3, is of interest for a detailed investigation. Table 3. PROMISING OZONE-FRIENDLY SUBSTITUTES ODP=0; GWP<300; (F/H+F)>65%
2. Fluorine-containing olefines In recent years FSUE RSC "Applied Chemistry" has carried out widespread investigations on improvement of commercial technologies for production of fluoroolefines such as tetrafluoroethylene, vinylidene fluoride and etc. The technology for production of commercial fluoroolefines was developed by pyrolysis of starting fluorochlorohydrocarbons in the presence of water vapor:
The conversion of the starting compounds and selectivity of the fluoroolefines in these processes considerably exceed the appropriate indices for "dry" pyrolysis process. Thus, in the pyrolysis of difluorochloromethane at 750-850oC the tetrafluoroethylene selectivity made 90-95% at difluorochloromethane conversion of 80%, while in the pyrolysis of 1,1-difluoro-1-chloroethane at 800-840oC the process selectivity towards 1,1-difluoroethylene was 98-99% at the almost full conversion of starting 1,1=difluoro-1=chloroethane. The new technology for tetrafluoroethylene production was implemented at Kirovo-Chepetsk chemical plant. The technology for fluoromonomers production for ion exchange membranes has been developed and improved. The perfluorinated membranes of Nafion type (DuPont, USA) are the most effective membranes at present for fuel cells. Investigations on creation of promising membranes for fuel cells and other applications derived from co-polymers of tetrafluoroethylene and perfluoro(-3,6-dioxa-4-methyl-7-octene)-sulfonyl fluoride are also under development by FSUE RSC "Applied chemistry". The overall scheme of the synthesis of this copolymer is given below:
On the basis of this copolymer are obtained by extrusion films, that are used for creating the ion exchange membranes. RSC "Applied Chemistry" conducts investigations on creation of highly effective fluorine-containing membranes due to the use in the copolymers of new fluoromonomers such as perfluoroalkylvinyl ethers and etc.. 3. Fluorine-containing inert dielectric liquids, oils, lubricants 3.1. The experts of RSC Applied Chemistry have developed a series of fluorine-containing dielectric liquids obtained by a standard scheme by the method of electrochemical fluorination [3]. Fluorine-containing liquids derived from perfluorinated tertiary amines have a wide range of application due to their unique properties: -a low solidification point (-100oC and lower), Someproperties of the perfluorinated dielectric liquids are given in Table 4. Table 4. Perfluorinated dielectric heat-transfer agents
Together with the fluorine-containing compounds, given in Table 4, the method of electrochemical fluorination is widely used in the developments of RSC "Applied Chemistry" for the synthesis of perfluoromonocarboxylic acids C2-C9 on the basis of various raw materials as well as for perfluorosulpho acids or their derivatives, for example perfluoroethylsulpho fluoride, perfluoro(ethylcyclohexyl sulphofluoride) and etc. 3.2. In RSC "Applied Chemistry" has been developed and implemented the technology of manufacture of oligomers of hexafluoropropylene oxide, unique in their properties,- perfluoropolyethers of general formula
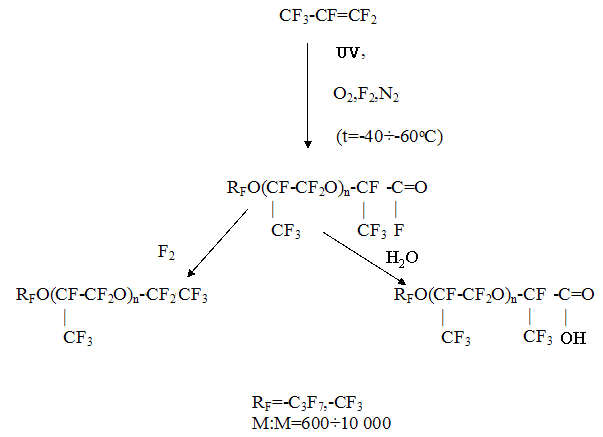
The technology of manufacture of these compounds consists in the oxidation of hexafluoropropylene (CF3-CF=CF2) with oxygen at low temperatures (-30oC and below) with initiation by UV, fluorine and etc. [3,15]. Scheme Synthesis of perfluoropolypropylene oxides
The stabilized oligomers obtained according to this method possess a wide range of the boiling temperature( from 50oC to 320oC) at 1 mm Hg in combination with the low freezing point (-60oC and below). Simultaneously the perfluoropolyethers possess the combination of unique properties- they are highly thermostable ( up to +450oC), chemically stable in highly aggressive media, nontoxic, they possess high dielectric characteristics, good lubricating qualities, radiation stability, their viscosimetric properties are little changed in the wide temperature range and others. The combination of the properties listed above makes it possible to use them in different areas of contemporary technology. Some properties of perfluoropolyethers are given in Table 5. Table 5. Some properties of perfluoropolyethers
4.Surfactants It is known that the fluorine-containing surfactants occupy key place among the known surfactants. Thus, the value of surface tension of derivatives of perfluoroenanthic, perfluoropelargonic acids are on the level of 15-16 dyn/cm. The low surface tension and also chemical and thermal resistance of fluorine-containing surfactants of different structure determined their wide application in the contemporary technology. In the RSC "Applied Chemistry" on the basis of perfluoroenanthic (C6F13COOH), perfluoropelargonic (C8F17COOH) acids and also on the basis of perfluoropropylene oxides of the formula
and sulpho-derivatives of the composition
the series of fluorine-containing surfactants of different structure has been developed: - Anion active surfactants (RFCOOMe, RFSO3Me etc.),
- Non-ionogenic surfactants [RFCONH(CH2CH2O)nH] Fluorine-containing surfactants have found wide application as bases for foaming agents- fire extinguishers for the extinguishing of burning petroleum products and polar liquids, additives for oils, diesel fuel for the purpose to reduce friction and increase the performances of machines and mechanisms, in microelectronics in the manufacture of semiconductor materials for cutting and grinding silicon plates, in the cleaning of articles, in galvanotechnics, etc. 5. Synthesis of fluorocompounds with the use of elemental fluorine Elemental fluorine has found wide application as the fluorinating agent in the synthesis of different fluorocompounds [16, 17, 18]. The industrial method of the synthesis of lower perfluoroalkanes has been developed: Csol+ F2gas The interaction of fluorine and graphite runs at a temperature of 550-650oC in two stages. In the first stage a layer of solid carbon polyfluoride (CFx)n is formed on the surface of graphite particles at a temperature of 550-600oC in the regime close to the adiabatic. In the second stage in the absence of fluorine is conducted the thermal decomposition of (CFx)n at a temperature of 600-650oC to form perfluoroalkanes. In order to provide thermal stabilization of the reaction zone the reactors with dynamic ( intermixed, moving) beds are used such as fluidized bed, ascending gas-dust flow and free falling graphite bed. As a result the mixture of gaseous reaction products of interaction of graphite and fluorine is obtained with the following composition: 40-45 wt% CF4, 20-25 wt.% C2F6, 15-20% C3F8, 10wt% C4F10. A number of low-tonnage technologies have been realized at the Experimental plant of RSC ?Applied Chemistry? on the fluorination of solids by elemental fluorine to obtain corresponding fluorides such as hexafluorides of selenium, tellurium and iridium, tetrafluorides of selenium and germanium: GeO2sol + F2gas Sesol + F2gas Sesoll + F2gas Tesol+ F2gas Irsol+ F2gas The production processes of these compounds were performed in multiple-heart reactors with productive
capacity up to hundreds of kilograms per year. The reactors were equipped with scanning thermocouples
that made it possible to observe the temperature distribution in the reactor, the heat removal
was accomplished by free convection. The technology of the synthesis of nitrogen trifluoride
by fluorination of NH4F*nHF melt with fluorine gas has been developed: NH4F*nHFliq + F2gas NF3gas + NH4F*(n+m)HFliq where n<< The fluorination is carried out in the gradient-free reactor with a high-speed mixer in the circulation loop, that makes it possible to remove heat effectively on the basis of forced convection and to support the isothermal conditions of carrying process as well as to ensure maximum contact surface of gaseous fluorine with the melt. With this method of conducting the process the productivity of the fluorination reactor reached 30 g NF3 *l-1 *h-1 at the fluorine conversion up to 90-95%.The concentration of nitrogen trifluoride in the synthesis gas attained 90%, nitrogen was the main admixture.The yield of difluoroamine, tetrafluorohydrazine and difluorodiazines did not exceed 2-3%. The technology of producing a number of inorganic fluorine-containing compounds which are used as oxidizers etc. has been developed with the use of elemental fluorine. The list of these compounds is represented in Table 6. Table 6. Inorganic fluorine-containing oxidizers
6. Fluorine-containing compounds for medicine In spite of high aggressiveness of initial fluorinating reagents, many fluorinated and perfluorinated compounds due to their unique physical and chemical properties are in particular of great interest for medicine and biology and are widely used all over the world as diagnostic and therapeutic preparations. Such compounds as perfluorodecalin, perfluorotributylamine, perfluorooctylbromide possess a unique ability to dissolve oxygen up to 45-50% by volume and taking into account their chemical inertness they are used as a gas-transport agents for various purposes: for creating the artificial blood substitute, in ophthalmology for treatment of the retinal detachment, in roentgenography etc. Some fluorocarbons used in this direction in medicine are represented in Table 7. Table 7. Fluorocarbons for medicine
Implementation of these compounds into medical practice is conducted together with medical institutions such as Military Medical Academy (Saint-Petersburg) and others. 1. V.G. Barabanov, O.V. Blinova, V.S. Zotikov, V.B. Rusanov, V.N. Tselikov, Rossijskaya natsional'naya strategiya upravleniya hladonami, Khimizdat, Sankt-Peterburg - Moskva, 2003, 56 s. 2. V.G. Barabanov, O.V. Blinova, V.S. Zotikov, S.A. Lizgunov, A.P. Orlov, G.D. Orlov, V.B. Rusanov, V.I. Samojlenko, I.G. Trukshin, V.N. Tselikov, Ozonobezopasnye al'ternativy i zameniteli. Propellenty, hladagenty, vspenivateli, rastvoriteli, ognegasyashchie sredstva, Khimizdat, Sankt-Peterburg – Moskva, 2003, 304 s. 3. B.N. Maksimov, V.G. Barabanov, I.L. Serushkin, V.S. Zotikov, I.A. Semerikov, V.P. Stepanov, N.G. Sagajlakova, G.I. Kaurova, Promyshlennye ftororganicheskie produkty: Spravochnik, Khimiya, Sankt-Peterburg, 1996, 544 s. 4. Patent RU № 2051140, 1993. 5. D.V. Vinogradov, V.A. Homutov, Kinetika i kataliz, 2000, 41,396. 6. Patent RU № 2052442, 1992. 7. Patent RU № 2065430, 1994. 8. Patent RU № 2051139, 1993. 9. Patent RU № 2005539, 1992. 10. B.N. Maksimov, V.G. Barabanov, ZhPKh, 1999, 72, 1944 11. J. Kijowski, G, Webb, and J. M. Winfield, J. Fluorine Chem., 1986, 27, 181. 12. I.G. Trukshin, V.M. Vishnyakov, I.E. Mitina, S.G. Fedorova, T.E. Kramerova, Tez. dokl. Mezhdunar. seminara «Novye holodil'nye sistemy», Sankt-Peterburg, 1995. 13. A.A. Bezdenezhnyh, Inzhenernye metody sostavleniya uravnenij skorostej reaktsij i rascheta kineticheskih konstant, Khimiya, Leningrad, 1973, 256 s. 14. A.V. Dmitriev, I.G.Trukshin, P.Yu.Smykalov, ZhPKh, 2002, 75, 789. 15. Patent RU № 2046127, 1993. 16. D.S. Pashkevich, Yu.A. Alekseev i dr. Vysokoekzotermicheskij sintez termostabil'nyh soedinenij v nestatsionarnom teplovom rezhime v reaktsionnoj sisteme gaz-tverdoe telo, ZhPKh, 1999, t.72, v.12, 2007- 2011. 17. Patent RU № 2115645, 1996. 18. D.S. Pashkevich, D.A. Muhortov i dr. Gazofaznoe ftorirovanie ftoretanov ftorom. ZhPKh, 2001, t. 74, v. 7, 1120-1125. |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fluorine Notes, 2009, 62, 3-4
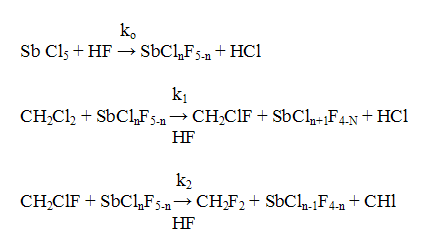
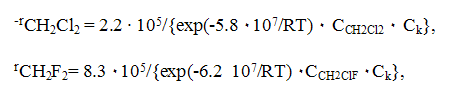
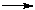 C
2H2ClF3 +2HCl + 93 kJ/Mol (1)
C
2H2ClF3 +2HCl + 93 kJ/Mol (1)