Fluorine Notes, 2009, 62, 1-2
Research on development of a technology of fluorine insertion into polymers by high-valent transition metal fluoridesaV.V.Kornilov, bYu.A. Malkanduev, aB.N.Maximov a FSUE "RSC "Applied Chemistry", 197198, Saint-Petersburg, 14,Dobrolubov
ave. A great number of different methods of polymers modification have existed including treatment with flame, plasmachemical modification, corona discharge, initiation by various methods such as co-graft polymerization, metallization, chlorination, sulfonation etc. In recent years surface modification of polymeric materials with the use of fluorinated reagents is of increased interest because the modified polymers possess unique characteristics, including thermal and chemical stability, good gas separating and high barrier properties towards liquids, heightened adhesion etc.[1-9]. Main developments in this field carried out in the USA, Belgium, SAR and China have been devoted to surface treatment of polymers with mixtures of elemental fluorine with various diluents [5-9]. This article considers investigations with the use of original methods of fluorine insertion into polymers by treatment with metal fluorides in liquid phase. By the middle 80-s of the last century the process of direct fluorination was widely used in industry for improvement of barrier properties of polymeric articles. A quite sufficient decrease for industrial application (50-100times) of the permeability for fuel and other non-polar liquids through walls of polymeric tanks and different reservoirs was achieved [1-9]. A vigorous nature of the reactions between elemental fluorine and hydrocarbons is well known. It evolves from weakness of F-F bond, strength of C-F bond, stability of splitted HF group and extremely low activation energy of hydrogen atom detachment and fluorine substitution. The reaction of fluorine with organic polymers is so exothermic that usually brings to the substrate fragmentation and destruction if the reaction is not controlled. The reaction conduction under milder conditions may be achieved by the following:
The latter effect can be realized by arrangement of a relatively passive surface layer by means of aging of the surface under low partial pressure of fluorine [1]. The earlier investigations studied fluorination of simple polymers such as polyethylene with the purpose to obtain perfluorinated materials [5]. The surface fluorination proceeds according to the free-radical mechanism when fluorine detaches hydrogen atoms from hydrocarbon and replacement for fluorine atoms occurs. Fluorine deactivates geminal hydrogen atom towards detachment, so the process running becomes more and more difficult. The process can be illustrated in the following way: Initiation stage:F2 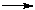 2F . 2F .
Stage of the chain growth: F . + -CH2-CH2- -C .H-CH2- + F2 Stage of the chain breaking: F . + F . -CH .- CH2-+-CH .- CH2-
F.+ -CH.- CH2- In this simplified reaction scheme the initiation proceeds at the expense of dissociation of the fluorine molecule at room temperature and pressure (the partial fluorine pressure is approximately 105 Pa). The initiation process may be accelerated by heating, polymer preheating or by heat or radiation application. Polyethylene surface fluorination was studied in article [9] and possible routes and probability of these reactions was discussed. A big number of the reaction products include partially fluorinated, perfluorinated, oxygenated and non-fluorinated moieties that may be linear, branched or cross-linked ones, fluorination of a wide scope of polymers has been studied. Complete fluorination was achieved for the most of them as a result of gradual increasing the partial pressure of fluorine to compensate its drop and to make hydrogen detachment easier. The attempts to model the reactions of surface fluorination of polymers were made repeatedly, though no one of them was completely successful. The experiments on polycarbonate, poly(methyl)methacrylate and polysterene have resulted in a conclusion that at room temperature the fluorination completion degree is controlled by gas diffusion inside polymer [5]. In case of the investigation of surface fluorinated polyethylene, the reaction is limited by the rate at low temperatures and by mass transfer or diffusion of the reacting gas at high temperatures [6]. With the purpose to make the conditions of fluorination of polymers milder this paper presents investigations on development a technology of obtaining modified samples of polyethylene by means of treatment with high-valent transition metal fluorides. EXPERIMENTAL In this work as a subject of the investigation were taken samples of polyethylene films ST and SM ( State Standard 10354-82) of 0.2 mm thickness, that have found a wide application in various branches of industry and produced on industrial scale. 1. Preparation and modification of polymers surface 1.1. Preparation of high-valent transition metal fluorides As metal fluorides there were used high-valent fluorides of cobalt (CoF3) and cerium (CeF4) obtained by the following treatment according to the scheme: 2MeFn + F2 For that a vertical tubular reactor made of nickel of 0,2 dm3 volume, equipped with electrical heating, was filled with 200g of the low-valent fluoride powder (CoF2 or CeF3 ), the reactor was sealed and pressurized with 0.1Mpa (overpressure) of nitrogen. The reactor containment was controlled by the absence of pressure drop for 2 hours. After that the overpressure was thrown off and the reactor was heating up to a temperature of 230-240oC. When the predetermined temperature was attained the feeding of elemental fluorine with the rate up to 2 dm3/hour was started. The reaction completion was registered by appearance of gas fluorine at the outlet from the reactor. After that the reactor was purged with gas nitrogen for 1 hour to remove gas fluorine traces, the reactor was cooled and the obtained modified higher metal fluoride (CoF3 or CeF4 accordingly) was poured into a metal container that was hermetically sealed. 1.2.Polymers fluorination Since high-valent transition metal fluorides are powders under normal conditions, the process was carried out in liquid phase in an inert perfluoroorganic liquid medium ( perfluoromethylcyclohexane, perfluorodecalin, perfluorooctane etc.). The investigations were carried out to find optimal conditions for fluorination of polymer samples with high-valent transition metal fluorides. Experiment methodology Before starting the experiments the reactor and pipe lines were degreased and after dried with compressed nitrogen. In the reactor of 0,3 dm3 volume equipped with a bubbler and a thermostating system a film sample of 25x50mm size was placed and poured with 200 mL of perfluoroorganic liquid. The samples were placed in such a way that they were above the bubbler and were completely immersed in the perfluoroorganic liquid. After that a necessary quantity of high-valent fluoride was added into the reactor, the reactor was closed, purged with helium to provide mixing. The gases leaving the reactor contained hydrogen fluoride in small quantity and were delivered to the neutralization system. The obtained samples were washed with isopropyl alcohol and dried in a desiccator for 24 hours. The fluorination temperature for polyethylene samples was 35, 50 and 80oC, the aging time was 5 hours. According to the results of the conducted investigation it was established that the treatment of polymers samples did not bring to destruction of the starting material. The obtained samples were tested for chemical resistance and thermal stability. The control of the surface and degree of fluorine atoms insertion into the polymeric samples were realized by means of IR-spectrometers (Bruker IFS-28) using combinations of absorption and reflectance IR-spectra in the analysis. In order to measure IR-spectra, the samples of 10x10mm size were cut out from the modified polymer and were fasten in the cuvette section at right angle to the beam. The analysis of the absorption spectrum was realized by means of OPUS software (version 6). A change in the intensity peak within a 730-719 cm-1 range corresponding to C-H bond and appearance of a new peak within a 1100-1300 cm-1 range corresponding to C-F bond were observed( Fig.1).
Fig.1. Standard IR spectrum of the polymer sample before (A) and after (B) modification The appearance of C-F bond peak is the evidence of the process of substitution of hydrogen for fluorine. The next stage studied FTIR-spectrum of absorption. For that a device with the modified polymer sample of 10x10mm size was placed in the cuvette section. A drastic decrease in the intensity of the C-H bond in the reflectance spectrum is the evidence of modification of the polymeric sample with substitution of hydrogen atoms on the surface by fluorine. The analysis of the IR-spectra of the modified polymeric samples has shown that the use of high-valent transition metal fluorides as the modifying reagents in finding the optimal process conditions allows both to limit to fluorination on the surface with creation of a protective fluorine-containing layer and to continue further insertion of fluorine atoms into the polymer matrix under more severe reaction conditions. 2. Evaluation of chemical resistance and thermal stability of modified polymer samples. The samples of starting and modified polymers were tested for oxidation stability The oxidation test consisted of 2 stages: a) preliminary preparation of the samples included 4 steps: - hydrolysis of the sample at 90oC for 1 hour in KOH/dimethylsulfoxide /water medium (3:6:11 by weight) - washing the sample with water; - aging for an hour at 90oC in 4N H2SO4 - washing the sample with water and drying b) evaluation of resistance to oxidation in a medium of 30% hydrogen oxide+20 ppm Fe2+(FeSO4) at 70 oC for 30 hours. The sample is considered to pass the test if it weight loss does not exceed 5%. After finishing the tests the samples were cooled to room temperature, washed with distilled water, dried with filter paper to remove drops completely and in a minute were weighted with an accuracy of 0.0002g. The test results are given in Table 1. Table 1. Oxidation stability of the samples of polymer materials modified with fluorine ( medium: 305 hydrogen oxide + 20 ppm Fe2+(FeSO4) )
For comparison, there are given results in the Table obtained for the starting material without modification ( 3 and 4 lines). Table 2 presents the results of thermal treatment of the samples. Table 2. Thermal stability of the treated samples of polymer materials
Samples of ST and SM fuse at 108-110 without treatment. Thus, it has been shown that the films modified by means of high-valent transition metal fluorides are stable to oxidation as well as to elevated temperatures. References |
1. Surface Fluorination of Polymers / Madhu Anand, J. P. Hobbs, Ian J. Brass in book "Organofluorine Chemistry: Principles and Commercial Applications" / edited by R.E. Banks, B.E. Smart and J.C. Tatlow, New York : Plenum, 1994
2. G.G. Furin, Ftoristyj vodorod. Ftoriruyushchij reagent i rastvoritel' v organicheskom sinteze.Novosibirsk, 2002,s. 35-342
3. A.P. Kharitonov. Kinetika i mekhanizm pryamogo ftorirovaniya polimerov. Dissertatsiya na soiskanie uchenoj stepeni doktora f.-mat. nauk. g. Chernogolovka, 2005
4. Lagow, R. J., J.L. Margrave, "Direct Fluorination: A New Approach to Fluorine Chemistry", Progress in Inorganic Chemistry, 26, pp. 161-210 (1979).
5. J. Jagur-Grodzinski. Modification of polymers under heterogeneous conditions//Progr. in Polymer Sci.-1992.-V.17.-P.361-415
6. Toy M.S. , J. Polym. Sci., Surface-polymerization of tetrafluoroethylene on fluorineactivated metal substrates, Part C, 1971, N 34, P 273
7. Momose Y., Takada T., Okazaki S., (Fac.Eng., Ibaraki Univ, Hitachi, Japan 316) Tetrafluoromethane and hexafluoroethane plasma fluorination of hydrocarbon and fluorocarbon polymers, J. Appl Polym Sci: Appl Polym Symp 1987 (pub 1988). 42 (Plasma Polym Plasma Treat Polym), 49-71,СА 109, 93775
8. Strobel M., Lyons C.S, Thomas P.A, Morgen M.C., Corn S., Korba G.A., J. Appl Polym Sci: Appl Polym Symp 1987 (pub 1988). 42 (Plasma Polym Plasma Treat Polym), 73- 96,СА 109, 93776J
9. Amouroux J.; Arefi F., Surface treatment of polymer films by a nonequilibrium plasma, Polym. mater Sci. Eng.,1990, 62, 418-22
Fluorine Notes, 2009, 62, 1-2