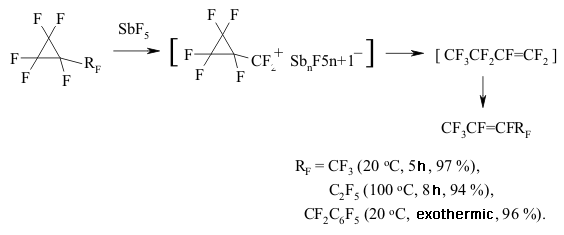Fluorine Notes, 2008, 61, 3-4
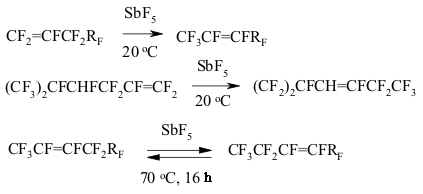
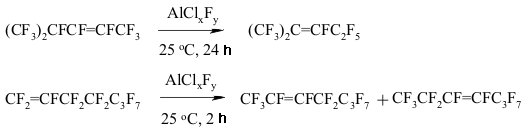
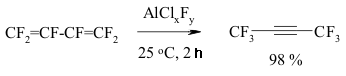
A BREAKTHROUGH IN CHEMICAL TECHNOLOGIES OF FORMING THE MULTIPLE BOND WITH FLUORINE ATOMS AND PERFLUOROALKYL SUBSTITUENTS AT ITA.N. Il'in*, G. G. Furin *614113, Russia, Perm, ul. Lasvinskaya, 98, JSV Halogen 7. The Migration of Double Bond in Fluoroolefines and Fluorodienes Catalyzed By Antimomy Pentafluoride The izomerization of perfluoroolefines can be carried out by transforming of carbocation generated for example by the SbF5 influencing perfluoroolefine. At that the terminal pefluoroolefines (perfluoropent-1-en, perfluorohex-1-en etc.) under the influence of catalytic quantitites of SbF5 are smoothly isomerized into corresponding trans-fluoroolefines with the multiple bond in the position 2 (yield is around 80-85%) [136-139]. The isomerization of perfluoro-4-methylpent-2-ene into perfluoro-2-methylpent-2-ene goes analogously to the one of perfluorobuta-1,3-dien into perfluorobut-2-en [140]. The isomerization of terminal perfluorolefines into internal ones is carried out stereoselectively and results in trans-isomers almost exclusively. Except for prefluoroallylbenzene, which under the influence of catalytic quantities of SbF5 is being isomerized into the mixture of cis- and trans-perfluoropropenylbenzenes at a rate of 1:1. Pure cis- and trans-perfluoropropenylbenzenes are again forming the mixture of isomers of the same proportion under the influence of SbF5 [141]. Other catalysts, for example AlClxFy, can be effective for that processes as well [142]. In that case, the reaction period is of great importance (Table 3) [140]. Thus, the isomerization of perfluorohept-1-en at catalysis of AlClxFy produces the mixture of perfluorohept-2-en and perfluorohept-3-en in one hour at a ratio of 65:35. Table 3. Perfluoroheptene-1 isomerization (catalyst AlClxFy) [140]
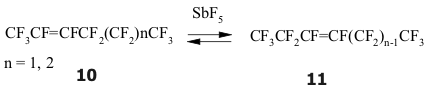
Perfluoroisopropylethylene is re-grouping into tris(trifluoromethyl)ethylene at heating up to 30-40 oC [137-139]. If a molecule has two terminal multiple bond, then they will be isomerized simultaneously, producing 80-90% E,E-isomers and 10-20% E,Z-isomers [143,144]. The boiling of perfluorohex-2-ene together with SbF5 leads to the forming of equilibrium mixture, containing of about 75-80 % of olefine 10 and of 20-25 % of olefine 11 [145]. All this shows us the presence of reversible 1,3-migration of fluorine in fluoroolefines at catalysis of SbF5.
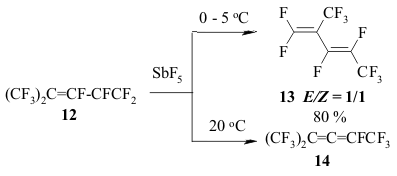
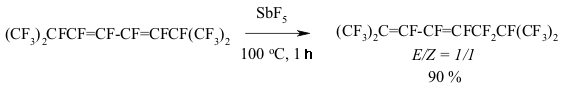
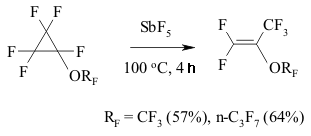
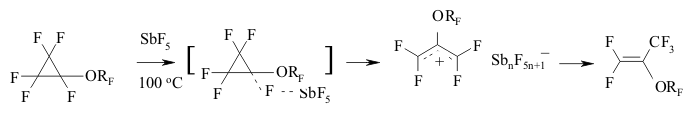
The transference of a multiple bond during the catalysis of SbF5 can be seen amongst perfluorinated diens as well [146]. Thus, perfluoro(2-methylpenta-2,4-dien) 12 is being isomerized into perfluoro(2-methylpenta-1,3-dien) 13 during the influence of SbF5 at temperature ranging from 0 to 5 oC. This compound is quantitively transforming into tris-(trifluoromethyl)fluoroallen 14 at 20-25 oC. The movement of both multiple bonds along the chain is not excluded during the isomerization of them both. The migration of double bond under the influence of SbF5 passes through the intermediate forming of perfluoroallyl cation or via the transitional condition which is like the allyl cation structure [147]. SbF5 play a key function here . A substituent at the allyl atom of carbon also influences the stereochemistry of the process. Thus, the isomerization of perfluorobenzene results in forming of a mixture containing cis- and trans-perfluoropropenylbenzenes, the decreasing of temperature of such isomerization increases the yield of trans-olefine (at -30 oC the ratio of trans/cis is 3/1) [147]. Trans-isomer is a product of kinetic control. At the same time for the perfluorinated conjugate dienes the forming of a triple bond is more likely than the isomerization of multiple bonds. Actually, during the influence of AlClxFy on perfluorobutadiene we obtain perfluorobut-2-ene with the quantitive yield [140]. Perfluorocyclopropanes can be isomerized by SbF5 as well. Thus, perfluoroalkoxicyclopetanes are isomerized under the influence of SbF5 at 100 oC into perfluoro(2-alkoxipropylene) [148]. The reaction is probably carried out according to the following scheme:
The generating of carbocation is registered according to NMR 19F spectra. It is rather interesting, that during the substitution of perfluoroalkoxy group for trifluoromethyl group the direction of reaction is changing significantly. For example, perfluoromethylcyclopropane and perfluoro(benzylcyclopropane) are being isomerized into perfluorobut-2-ene and perfluoro(1-phenylbut-1-ene) under the influence of SbF5 at as low temperature as 20 oC, while the temperature needed to transform the perfluoro(ethylcyclopropane) is equal to 100 oC [148].
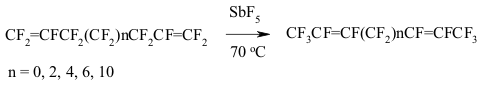
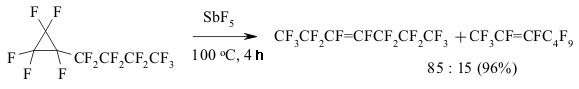
The forming of perfluorolefines mixture is possible due to the isomerization of a multiple bond. Thus, perfluorobutylcyclopropane produces the mixture of perfluorohept-3-ene and perfluorohept-2-ene under the influence SbF5of at temperature of 100 oC [148]. 8. Synthesis of Perfluorinated Cyclic Compounds out of Fluorinated Dienes and Polienes Under the influence of SbF5 perfluorinated poliens are being subject to intramolecular cycloaddition forming tetra- or penta-member cycles [143,144].
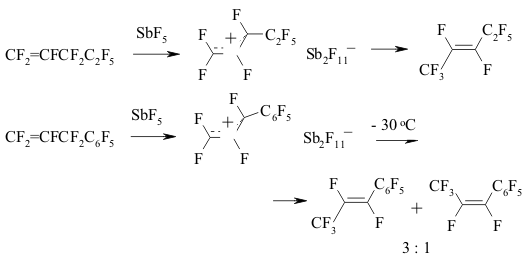
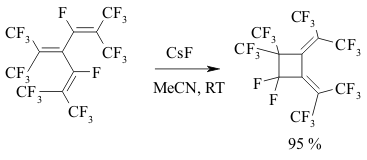
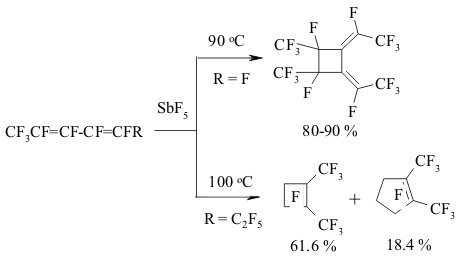
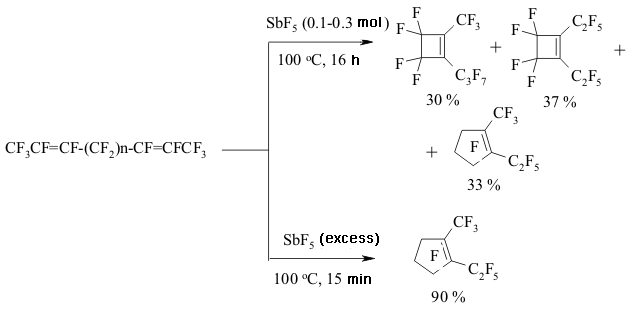
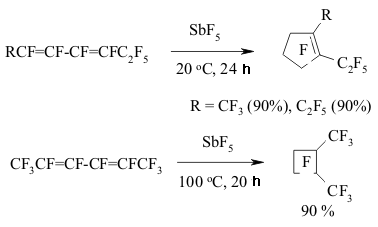
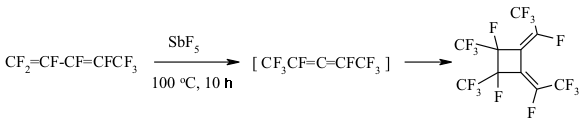
The implementing of intramolecular cyclization leading to the forming of perfluorodialkylcyclopentenes is an important moment of perfluorinated dienes transformations in the medium of SbF5 as German and his coworkers proved [142,146,149-151]. At that the quantities of SbF5 are very important. Conjugate perfluorinated dienes over SbF5 also transform into the derivatives of cyclopentene under the mild conditions. However, if a conjugate diene contains only CF3 groups, that at room temperature the cyclic product will not be formed, whereas by increasing the temperature up to 100 oC perfluoro-1,2-dimethylcyclobutene is formed [146]. At the same time the presence of only one terminal CF3 group results in forming of cyclobutane system [146]. The isomerization of perfluoropenta-1,3-dien to perfluoro-1,3-dimethylallen which is further cyclodimerized, may take place in SbF5 .
Conclusion The material listed above allows establishing the on growing researchers' interest for the problem of updating the tetrafluorethylene production method and for the working out the approaches of its transforming into hexafluoropropylene and other perfluororganic compounds. The significant results achieved here prove, that they in a number of cases can become an alternative for the well-known methods. We should mention a wide application of ion-fluoride as a catalyst for the isomerization processes, which uncovers a possibility for synthesis of perfluoroolefines of different structure. Such processes have got obvious advantages and real opportunities for the application in industrial technologies. At implementing of that methodology a discovery of new reactions and transformations resulting in synthesis of fluorine containing compounds should be expected. There is no doubt that issues regarding the implementing of new approaches and ideas are of interest of not only chemists working in the filed of fluoroorganic compounds chemistry but of specialists in the field of organic synthesis. At that it is obvious, that in a number of cases perfluoroorganic compounds are convenient and sometimes unique models for setting and solving some fundamental issues of theoretical organic chemistry. References 1. Organofluorine Chemistry, Principles and Commercial Applications. Eds. Banks R.E., Smart B.E., Tatlow J.C..Plenum Press. New York. 1994, p. 287. 2. Novoe v tekhnologii soedinenij ftora. Pod red. Isikawa N., M.: Mir. 1984, 591c. 3. Banrs E.R. Fluorine in agriculture fluorine technology. Manchester : Fluorochem. Ltd., 1994. 4. Walker S.B. Fluorine compounds as agrochemicals. Old Glossop (U.K.) : Fluorochem. Ltd., 1990. 5. Chemistry of organic fluorine compounds II. Ed. M. Hudlicky, A.E. Pavlatt. Washington (DC) : Amer. Chem. Soc. 1995, 1296p. (ACS Monogr.; N 187). 6. Synthetic fluorine chemistry. Eds. G.A. Olah, R.D. Chambers, G.K.S. Prakash. N.Y. : WileyIntersci. Publ. 1992, 480p. 7. Fluorine in bioorganic Chemistry. Eds. J.T. Welch, and S. Eswarakrishnan.Wiley : New York, 1991. 8. Furin G.G. Ftorsoderzhashchie geterotsiklicheskie soedineniya. Sintez i primenenie. Novosibirsk : Nauka. 2001, 304c. 9. Furin G.G., Fainzil'berg A.A. Sovremennye metody ftorirovaniya organicheskih soedinenij. M. : Nauka, 2000, 239 c. 10. Welch J.T., Eswarakrishnan S. Fluorine in Bioorganic Chemistry, Wiley, New York, 1991. 11. Filler R., Kobayashi Y. Biomedical Aspects of Fluorine Chemistry, Elsevier Biomedical Press, Kodansha, Tokyo, N.Y., 1982. 12. Sheppard U., Sharts K. Organicheskaya khimiya ftora. M.: Mir, 1972, 480c. 13. Bergstrom D.E., Swartling D.J.. in Fluorine-Containing Molecules. Structure, Reactivity, Synthesis and Applications. Eds. Liebman J.F., Greenberg A., Dolbier W.R.; VCH: New York, 1988, p. 259-308; 14. Organofluorine compounds in medicinal chemistry and biomedical applications./Eds. Filler R., Kobayashi Y., Yagupolskii K.M., Elsevier Science Publishers B.V., Amsterdam, Netherlands, 1993, 386p. 15. Furin G.G., Chi K.-W. Synthetic methods for fluoroorganic compounds. UUP, Korea, 2001, 400p. 16. Burger K., Wucherpfennig U., Brunner E.. Adv. Heterocycl. Chem.,1994, v. 60, p. 1. 17. Chambers R.D., Vaughan J.F.S. In : Topics in Current Chemistry. Chambers R.D. (Ed.), Springer-Verlag: Berlin, Heidelberg, New York, 1997, v. 192, p. 1-38. 18. Furin G.G. Adv. Heterocyl. Chem., 2004, v. 86, p. 129-224. 19. Furin G.G. Adv. Heterocyl. Chem., 2004, v. 87, p. 273-387. 20. Furin G.G. Adv. Heterocyl. Chem., 2005, v. 88, p. 239-320. 21. Barabanov V.G., Ozol S.I. Piroliticheskie sposoby polucheniya ftorsoderzhashchih olefinov. «TEZA» : S. Peterburg, 2000, 200c. 22. Schirmann J.P., Hub S., Lantz A., Lacroix E. Patent FR 2742434 , 1997. 23. A.N. Il'in, L.K. Limonova, G.R. Borzunov, i dr. Patent SU 270533. Publ. 1986. 24. Il'in A.N., Krasnov A.P., Limonova L.K., i dr. Patent SU. 211103, Publ. 1984. 25. Il'in A.N., Krasnov A.P., Limonova L.K., i dr. Patent SU 2151737, Publ. 1985. 26. Uklonskij I.P., Denisenkov V.F., Il'in A.N.,i dr. Pol. Reshenie 2000 111832/04(0128) ot 10.01.2001. 27. Uklonskij I.P., Denisenkov V.F., Il'in A.N., Mineev S.N. Pol. Reshenie 2000 121911/04(023392) ot 1.02.2001. 28. Il'in A.N. // Tezisy dokladov 2-oj Mezhdunarodnoj konferentsii «Khimiya, tekhnologiya i primenenie ftorsoedinenij», 23-26 sentyabrya 1997. Sankt-Peterburg. Rossiya, R2- 17, c. 88. 29. Nakahara A., Tokunaga S. Patent JP 07233104. 30. Golubev A.N., Dedov A.S., Denisov A.K., i dr. Patent RU 2136652, Publ. 10.9.99. 31. Drozhdin B.I., Dedov A.S., Zaharov V.YU., i dr., Patent RU 2150456 Publ. 10.06.2000. 32. Politanskij S.F. VkhO im. D.I. Mendeleeva, 1979, t. 24, s. 529. 33. Mel'nikovich S.V., Moin F.B. i dr. Kinetika i kataliz, 1984, t. 25, № 4, s. 993. 34. Kushina I.D., Bel'ferman A.L. Kinetika i kataliz, 1972, t. 13, № 4, s. 843. 35. Kushina I.D., Politanskij S.F., Shevchuk V.U. i dr. Izv. AN SSSR. Ser. khim., 1974, № 4. s. 946. 36. Barabanov V.G., No L.V., Fedurtsa M.U., Kushina I.D. // Zhurn. obshch. khimii. 1984. T. 54. s. 824. 37. Hayashi T. et al. Proc. Int. Ion Eng. Congr. Edited by T. Toshinori. Kyoto. Japan, 1983, N 3. p. 1611. 38. Webster J.L. Pat. 5633414 USA. Publ. 1997;РЖХим. 1997. 15Н 117П. 39. WO 371747 US. Publ. 1990. 40. Baddour R.F., Bronfin B.R. Ind. Eng. Chem. Proc. Design and Develop., 1966, p. 472. 41. Patent SU 168074. Publ. 1985. 42. Patent SU 200952. Publ. 1984. 43. Herman I.P. et. al J. Chem. Phys., 1980, v. 84, N 1, p. 516. 44. Shin J.C. et. al J. Photochem. Photobiol., A, 1993, v. 71, N 1, p. 9. 45. Brown C.E. et. al Proc. SPIE – Int. Soc. Opt. Eng. 1996. Vol. 669 (Laser Appl. Chem.) P. 52. 46. Hackett P.A. et. al J. Phys. Chem., 1980, v. 84, N 15, p. 1873. 47. Kato S. et. al J. Phys. Chem., 1984, v. 88, N 18, p. 3977. 48. Kato S. et. al Roza Kagaku Kenkyu, 1983, v. 83, N 5, p. 114. 49. Doyle D.J. et. al Polym. Prepr. (Amer. Chem. Soc. Div., Polym. Chem.), 1987, v. 28, N 1, p. 250. 50. Pola J. et. al J. Fluorine Chem., 1990, v. 50, p. 309. 51. Sviridova A.P. i dr. Khim. fizika, 1989, t. 8, № 6, s. 762. 52. Chowdhury P.K. et. al J. Phys. Chem., 1988, v. 92, N 1, p. 102. 53. Sugita K. et. al Reza Kagaku Kenkyu., 1992, v. 14, p. 84. 54. Trott W.M. et. al J. Photochem., 1984, v. 24, N 2, p. 4278. 55. Ichida T., Homoto Y.,Заявка 1481957 (2004)ЕПВ,МПК7С 07С 17/367 (Daikin Ind., Ltd.);РЖХим., 05.08-19Н.80П. 56. Downing F.B., Benning A.F., Mc Harness R.C., Patent US 2480560 (1949). 57. Il'in A.N., Polunina A.D., Krasnov A.P. Patent SU 272157 Publ. 1987. 58. Il'in A.N., Ivanova L.M., Tryastsina L.A.. 2-oj mezhdunarodnoj konferentsii «Himiya, tekhnologiya i primenenie ftorsoedinenij» 23-26 sentyabrya 1997. Sankt-Peterburg. Rossiya. Tezisy dokladov. P2-18, s. 89. 59. Sonoyama N., Ezaki K., Fujii H., Sakata T. Electrochimica Acta, 2002, v. 47, p. 3847-3851. 60. Schizodimou A., Kyriacou G., Lambrou C.H. J. Electroanal. Chem., 1999, v. 471, p. 26. 61.Probst A., Werner K.V. J. Fluorine Chem., 1990, v. 47, p. 163 62. Fleming G.L., Haszeldine R.N., Tipping A.E. J. Chem. Soc., Perkin Trans, 1, 1973, p. 574. 63. Deev L.E., Nazarenko T.I., Pashkevich K.I. Izv. Akad. Nauk SSSR. Ser. Khim., 1988, p. 402. 64. Tortelli V., Tonnelli C. J. Fluorine Chem., 1990, p. 199. 65. Baum K., Malik A.A. J. Org. Chem., 1994, v. 59, p. 6804. 66. Cirkva V., Paleta O., Ameduri B., Boutevin B. J. Fluorine Chem., 1995, v. 75, p. 87-92. 67. Barabanov V.G., Ozol S.I., Zajtsev S.A. WO 93002038/04 , Publ. 1996. 68. Manogue W.H., Rao M. Patent US 5892135. Publ. 1999. 69. Golubev A.N., Shabalin D.A., Novikova M.D., Murin A.V., Arasianov G.G., Lyubimova L.A., Lyubimov I.A., Tsarev V.A., Abramov O.B., Maslyakov A.I., Zakharov V.Yu., Dedov A.S. Russ Ru 2211209 (2003); Chem. Abstr., 2004, v. 140, 338958w. 70. Sekiya A., Yamada T., Sugawara M. Pat. 9743233. PCT Int. Appl. WO. Publ. 1997; Chem. Abstr., 1999, v. 128, 3450. 71. Cherstkov V.F., Galahov M.V., Mysov E.I., i dr. Izv. AN SSSR. Ser. Khim., 1989, № 6, c. 1336-1340. 72. Patent SU 226430. Publ. 1985. 73. Il'in A.N., Gavrilov V.G., Krasnov A.P., i dr. Patent SU 213773 Publ. 1983. 74. Il'in A.N., Gavrilov V.G., Krasnov A.P. III Ural'skogo seminara po khimicheskim reaktsiyam i protsessam v rasplavah elektrolitov. Perm'. 1982. Tezisy dokl. s. 59-60. 75. Moon D.J., Chung M.J., Kwon Y.S., Ahn B.S. Patent US 6710215 (2004) (Korea Inst. Of Science and Technology). 76. Krasnov A.P., Gavrilov V.G., Il'in A.N., Trubnikova T.F. Patent SU 168944. Publ. 1980. 77. Il'in A.N., Ivanova L.M., i dr. 2-ya mezhdunarodnaya konferentsiya «Khimiya, tekhnologiya i primenenie ftorsoedinenij». 23-26 sentyabrya 1997. Sankt-Peterburg. Rossiya. Tezisy dokl. s. 89. 78. Kotenko A.A., Novikova M.D., Amirhanov D.M., Zaharov V.Yu. Seminar «Ftorpolimernye materialy : fundamental'nye, prikladnye i proizvodstvennye aspekty». Istomino, ozero Bajkal. 9-11 avgusta, 2003. Tez. Dokl. UD-10. s. 55. 79. Moon D.J., Chung M.J., Kim H., et al. Ind. Eng. Chem. Res., 2002, v. 41, N 12, p. 2895- 2902; Chem. Abstr., 2002, v. 137, 79266. 80. Buravtsev N.N., Grigor'ev A.S., Kolbanovskij Yu.A. Kinetika i kataliz, 1985, t. 26, № 1. c. 7. 81. Drennan G.A., Matula R.A. J. Phys. Chem., 1968, v. 72, N 10, p. 3462. 82. Politanskij S.F. Kinetika i kataliz, 1969, t. 10, № 3, c. 500. 83. Kushina I.D., Fedurtsa M.U., Gida V.M., i dr. Izv. AN SSSR. Ser. Khim., 1989, № 6, s. 1433-1436. 84. Kushina I.D., Fedurtsa M.U., Barabanov V.G. i dr. V kn. V-aya Vsesoyuznaya konferentsiya po khimii ftororganicheskih soedinenij. Tez. Dokl. M. : AN SSSR. 1986, s. 146. 85. Buravtsev N.N., Kolbanovskij Yu.A., Ovsyannikov A.A. 1-ya mezhdunarodnaya konferentsiya «Khimiya, tekhnologiya i primenenie ftorsoedinenij v promyshlennosti». Tezisy dokl. Rossiya. Sankt-Peterburg. 1994, s. 183. 86. Buravtsev N.N., Kolbanovskij Yu.A. 2-ya mezhdunarodnaya konferentsiya «Khimiya, tekhnologiya i primenenie ftorsoedinenij». 23-26 sentyabrya 1997. Sankt-Peterburg. Rossiya. Tezisy dokl. 1997, s. 20. 87. Mallikarjuna R.V., Subramania M.A. Pat. 6031141 US (2000); Chem. Abstr., 2000, v. 132, 166716g. 88. Filyakova T.I. Zh. Org. Khim., 1991, v. 27, N 9, p. 1824-1828; Chem. Abstr., 1992, v. 116, 173512k. 89. Gorbunova T.I., Zapevalov A.Ya., Saloutin V.I. Russ. J. Org.Chem., 1999, v. 35, N 11, p. 1557-1566; Chem. Abstr. 2000. Vol. 133. 39506b. 90. Belen'kij G.G., Petrov V.A., Postovoj S.A., i dr. Patent RU 2210559, Publ. 1999. 91. Kremlev MM., Yagupolskii L.M. J. Fluorine Chem., 1998, v. 91, p. 109-123. 92. Resnick P.R. Pat. 11228474. Jpn. Kokai Tokkyo Koho JP. Publ. 1999; Chem. Abstr., 1999, v. 131, 170086. 93. Farnham W.B., Yang Z.-Y. Pat. 2000024709. PCT Int. Appl. WO. Publ. 2000; Chem. Abstr, 2000, v. 132, 294558. 94. Kashiwagi K., Murofushi H., Sugiyama N., et al. WO 1160231. Publ. 2001. 95. Mahmutov F.A., Tsareva E.I., Shebarshinova M.G. Patent RU 224704 (2004). 96. Miki J., Yoshimi H., Aoyama H. Pat. 6610896 U.S. (2003). 97. Burdenuic J., Crabtree R.H. J. Am. Chem. Soc., 1996, v. 118, p. 2525-2526. 98. Brooke G.M., Hall D.H., Shearer H.M.M. J. Chem. Soc., Perkin Trans. I, 1978, p. 780. 99. Brooke G.M., Hall D.H. J. Fluorine Chem., 1982, v. 20, p. 163. 100. Igumnov S., Lekontseva G.I. Patent US 6664431 (2003). 101. Fritz C.G., Selman S., Patent US 3291843 (1966). 102. Guerra M. Pat. 6624328 U.S. (2003) (3M Innovative Prop. Co.). 103. Fritz C.G., Moore E.P., Pat. 707360 Cand. (1965). 104. Henne A., Newby T. J. Am. Chem. Soc., 1948, v. 70, p. 130. 105. Hu C.-M., Liu H., Xu Z. J. Fluorine Chem., 1990, v. 46, N 3, p. 491-506. 106. Fialkova T.I., Kodess M.I., Pekhanskij N.V., i dr. Zhurn. organ. khimii, 1987, v. 23, N 9, p. 1858-1866; Chem. Abstr., 1988, v. 109, 37476e. 107. Chambers R.D., Lindley A.A. J. Fluorine Chem., 1978, v. 12, N 4, p. 337-340. 108. Farnham W.B. Chem. Rev., 1996, v. 96, p. 1633-1640. 109. Chambers R.D., Fuss R.W., Spink R.C.H., et al. J. Chem. Soc., Perkin Trans, 1, 2000, p. 1623-1638. 110. Moldavskij D.D., Furin G.G. Zhurn. obshch. khimii, 1996, t. 66, v. 12, s. 1995-2002. 111. PatentUS 3749793. Publ. 1973; Chem. Abstr., 1973, v. 79, 108060g. 112. Snegirev V.F., Makarov K.N. Izv. AN SSSR. Ser. Khim., 1985, № 9, s. 2066-2076. 113. Graham D.P. J. Org. Chem., 1966, v. 31, N 3, p. 955-957. 114. Chambers R.D., Jackson J.A., Partington S., et al. J. Fluorine Chem., 1975, v. 6, p. 5. 115. Narita T., Hagiwara T., Hamana H, et al. // Macromolecules. 1989. Vol. 22. P. 3183. 116. Chambers R.D., Taylor G., Powell R.L. J. Chem. Soc., Perkin Trans I, 1980, N 2, p. 429- 434. 117. Rakhimov A.I., Kaluga V.I. Zh. Org. Khim., 1980, v. 16, p. 223; Chem. Abstr., 1980, v. 92, 214861e. 118. Chambers R.D., Gribble M.Y., Marper E. J. Chem. Soc., Perkin Trans I, 1973, N 16, p. 1710-1715. 119. Chambers R.D., Taylor G., Powell R.L. J. Chem. Soc., Perkin Trans I, 1980, N 2, p. 426- 428. 120. Pruett R.L., Bahner C.T., Smith H.A. J. Am. Chem. Soc., 1952, v. 74, N 7, p. 1638-1642. 121. Chambers R.D., Taylor G., Powell R.L. J. Chem. Soc., Chem. Commun., 1978, N 10, p. 433. 122. Chambers R.D., Gribble M.Y., Marper E. J. Chem. Soc., Perkin Trans I, 1973, N 16, p. 1710-1711. 123. Chambers R.D., Nakamura T. 15th Symposium on Fluorine Chemistry. Tokyo. Japan. Oct. 22-23. 1990. Abstr. O-26. p. 88-89. 124. Zapevalov A.Ya., Filyakova T.I., Peschanyj N.V., i dr. // Zhurn. organ. khimii. 1990. T. 26. V. 2. S. 265-272. 125. Coe P.L., Sellars A., Tatlow J.C. J. Fluorine Chem., 1983, v. 23, N 2, p. 103-114. 126. Chambers R.D., Lindley A.A., Philpot P.D., et al. J. Chem. Soc., Perkin Trans, 1, 1979, N 1, p. 214-219. 127. Briscoe M.W., Chambers R.D., Mullins S.J., et al. J. Chem. Soc., Chem. Commun,. 1990, N 16, p. 1127-1128. 128. Chambers R.D., Nakamura T. J. Fluorine Chem., 1991, v. 54, N 1-3, p. 257-258. 129. Chambers R.D., Matthews R.S., Taylor G., Powell R.L. J. Chem. Soc., Perkin Trans I, 1980, N 2, p. 435-439. 130. Lin Y.D., Liang Y.D. Youji Huaxue, 1983, N 2, p. 97-100; Chem. Abstr., 1983, v. 99, 70152u. 131. Lin Y.D., Liang Y.D. Acta Chemica Sinica, 1981, v. 39, p. 654. 132. Dresdner R.D., Tlumac F.N., Young J.A. J. Org. Chem., 1965, v. 30, p. 3524. 133. Martini T., Halasz S.P. Tetrahedron Lett., 1974, p. 2129. 134. Krespan C.G., Dixon D.A. J. Org. Chem., 1998, v. 63, p. 36. 135. Krespan C.G. J. Fluorine Chem., 1994, v. 66, p. 311. 136. Krespan C.G., Petrov V.A. Chem.Rev., 1996, v. 96, p. 3269-3301. 137. Belen'kij G.G., Savicheva G.I., Lur'e E.P., German L.S. Izv. AN SSSR. Ser. Khim., 1978, № 7, s. 1640-1643. 138.German L.S. The seventh regular meeting of Japanese-Soviet Fluorine Chemists. Fukuoka. 1991. Abstr., v. 2, p. 19-1 – 19-10. 139. Petrov V.A., Belen`kii G.G., German L.S. 1th All-Union Conference on Organofluorine Chemistry. Tashkent. Abstr., 1982, p. 202. 140. Petrov V.A., Krespan C.G., Smart B.E. J. Fluorine Chem., 1996, v. 77, p. 139-142. 141. Belen'kij G.G., Savicheva G.I., Lur'e E.P., German L.S. Izv. AN SSSR. Ser. Khim., 1978, s. 1640-1643. 142. Petrov V.A., Krespan C.G., Smart B.E. J. Fluorine Chem., 1998, v. 89, p. 125-130. 143. Patent JP 60 23 33. Kokai Tokkyo Koho JP. Publ. 1985; Chem. Abstr., 1985, v. 103, p. 5882. 144. Probst A., Raab K., Ulm K., Werner K. J. Fluorine Chem., 1987, v. 37, p. 223. 145. Smart B.E., Middleton W.J., Farnham W.B. J. Am.Chem. Soc., 1986, v. 108, p. 4905. 146. Petrov V.A., Belen'kij G.G., German L.S. Izv. AN SSSR. Ser. Khim., 1989, № 2, s. 385-391. 147. Petrov V.A., Belen'kij G.G., German L.S. Izv. AN SSSR. Ser. Khim., 1982, № 10, s. 2411. 148. Chepik S.D., Petrov V.A., Galahov M.V., i dr. Izv. AN SSSR. Ser. Khim., 1990, № 8, s. 1844-1851. 149. Filyakova T.I., Belen'kij G.G., Lur'e E.P., i dr. Izv. AN SSSR. Ser. Khim., 1979, № 3, s. 681-683. 150. Petrov V.A., Belen'kij G.G., German L.S. Izv. AN SSSR. Ser. Khim., 1981, № 8, s. 1920-1922. 151. Belen'kii G.G., German L.S. Chem. Rev. (Soviet Scientific Reviews), 1984, v. 5, p. 206. |
|||||||||||||||||||||||||||||
Fluorine Notes, 2008, 61, 3-4