Fluorine Notes, 2008, 60, 3-4
A BREAKTHROUGH IN CHEMICAL TECHNOLOGIES OF FORMING THE MULTIPLE BOND WITH FLUORINE ATOMS AND PERFLUOROALKYL SUBSTITUENTS AT IT
A.N. Il'in*, G. G. Furin *614113, Russia, Perm, ul. Lasvinskaya, 98,
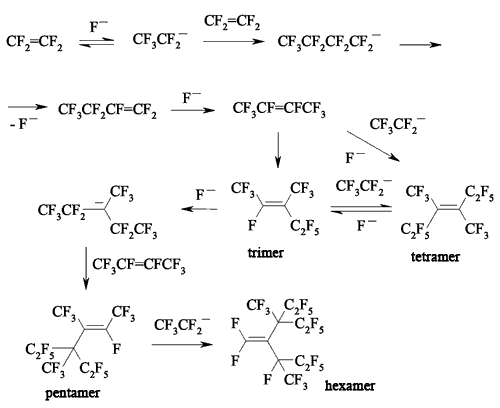
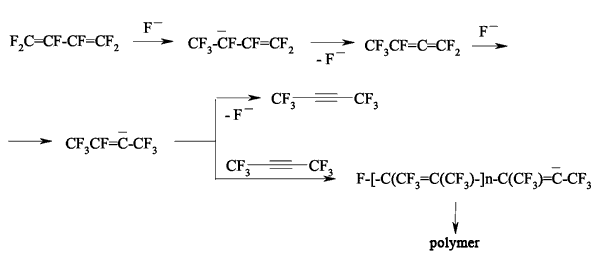
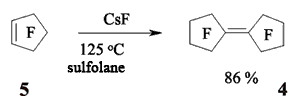
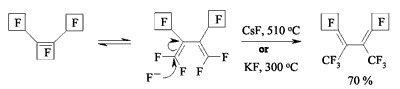
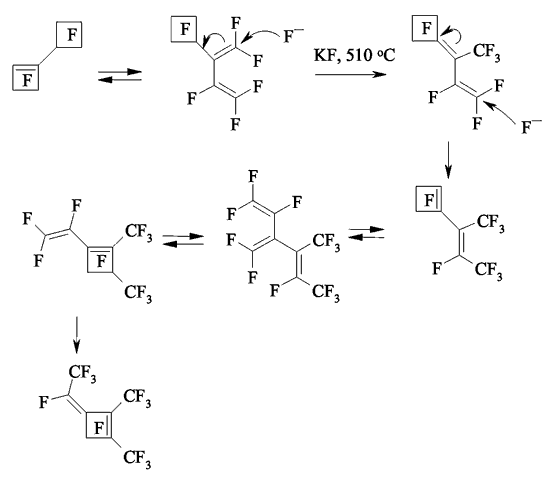
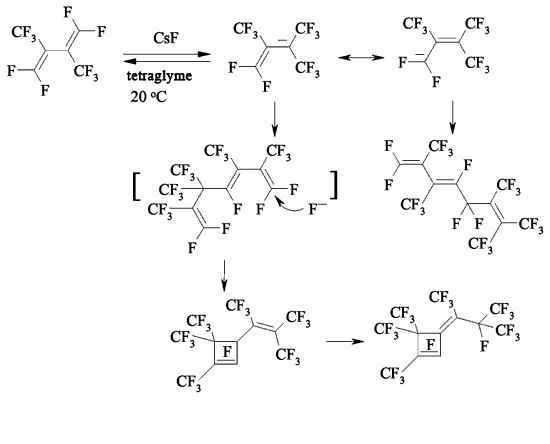
JSV Halogen (continuation) 6. Oligomerization of Perfluorolefines with Fluoride-Ion Participating As fluoride-ion is eliminated during the reaction of perfluorolefins and nucleophilic reagents, this provokes the process of oligomerization of both initial perfluorolefine and olefine isomerizated by fluoride-ion. Thus, fluoride-ion catalyzes the oligomerization of tetrafluorolefine, resulting in forming of olygomers [110-112]. The oligomerization of perfluorolefines and perfluorocycloolefines under the influence of fluoride-ion is well known [113,114]. Thus, the oligomerization of tetrafluoroethylene produces the mixture ranging from trimer to hexamer due to the generation processes of quite active carbanions and due to the isomerization processes of intermediate perfluorolefines. The oligomerization of hexafluoropropylene under the influence of fluoride-ion and over the crown ether goes by the analogous pattern. Thus, perfluoro(4-methyl-3-isopropylpent-2-ene) and perfluoro(3-ethyl-2,4-dimethylpent-2-ene) were obtained out of hexafluoropropylene under that conditions. In case of hexafluorobuta-1,3-diene fluoride-ion catalyzes the polymerization process [115]. Besides the polymer, the compound named hexafluoro-but-2-ine was isolated as well. That allowed the authors to suggest the following scheme of transformations: The oligomerization of perfluorocycloalkenes goes according the analogous scheme. Here the mixtures of dimers are formed as a rule and the reactions are quite sensitive to the conditions of carrying out the process. Thus, perfluorobicyclophenylidene 4 is formed out of perfluorocyclopentene 5 by CsF acting in sulfolane at 125 oC [116] or during the heating at that temperature for 20 hours (yield is 86 %) [117].
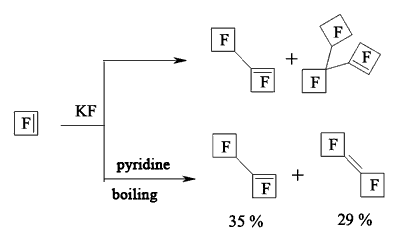
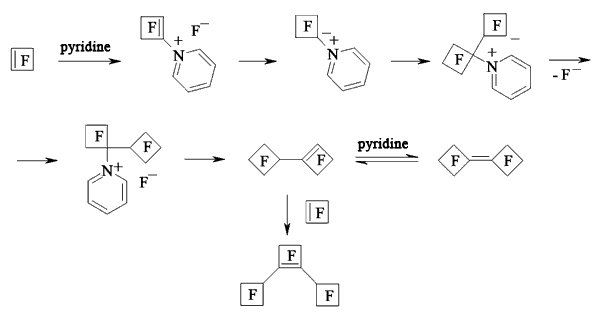
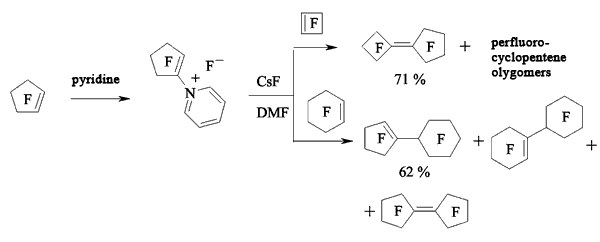
In case of perfluorocyclobutene the yield of dimers increases when using pyridine as a solvent [116,118-120].
At first, when pyridine is influencing perfluorocyclobutene the salt which contains active cyclobutenylic anion is formed [119,121]. When the perfluorocyclobutene is in excess or in case of adding another perfluorolefine to the reaction mixture the reaction replacing fluorine atom at multiple bond takes place.
The rate of formed mono-and di-substituted products depends on the amount of pyridine. Thus, at mole rate the initial olefine : pyridine = 15 : 1 the yield of mono-substituted products amounts to 64%, while at the rate of 9 : 1 it is only 21%. Other perfluorocycloolefines follow the same way [122,123].
That processes are laid as a base for synthesis of a number of new dienes based on perfluorocyclobutene and [123]. Along with that it can be an obtaining method of corresponding dienes [124].
Fluoride-ion catalyzes the dienes forming process starting from perfluorocyclobutene [74].
The transformation of perfluorinated dienes into perfluorinated cyclic dienes under the influence of fluoride-ion was observed [125].
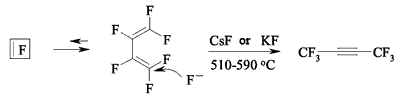
It should be noted, that the isomerization of perfluorocyclobutene under the influence of fluoride-ion can lead to the forming not of dimerization products, but of hexafluorobut-2-ine [126].
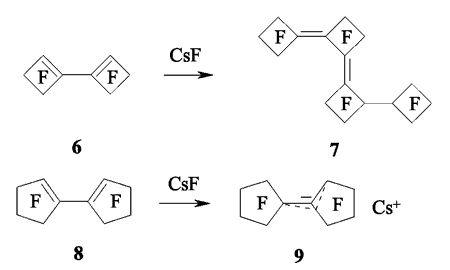
However, the use of CsF doesn't always provoke the oligomerization. Thus, if diene 6 transforms into triene 7 [127,128], then diene 8 produces only the corresponding salt 9 under the influence of CsF [129].
The oligomerization of perfluoroolefines can occur over other
catalysts as well. Thus, the authors of work [130,131] proved,
that
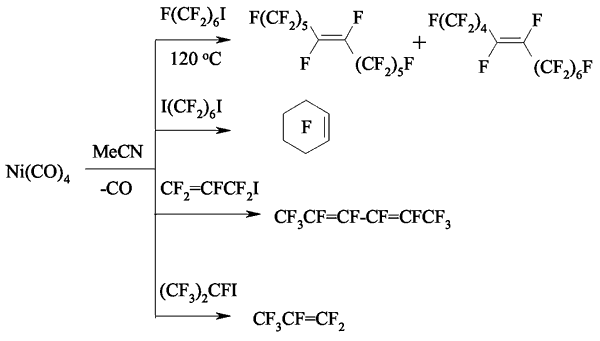
Later [134] it was proved, that carrying out of these reactions in donor solvents can result in appearance of metal-organic compounds with perfluoroalkyling properties. For example, at interaction of perfluoroalkyliodides and nickel tetracarbonyl at 25-60 oC in acetonitryl either dimer products or unsaturated compounds are formed [135].
Probably, at first the intermediate nickel compound is formed. At that the reaction of perfluoroallyliodide and nickel tetracarbonyl is an exothermic one. The nickel compound reacts with initial substrate producing the mixture of perfluorohexa-2,4-diene isomers. If we heat up the product of the 1,5-diiodperfluorohexane's and nickel tetracarbonyl interaction at the temperature over 120 oC, then we'll get two olefins of high yield that is the mixture of trans-perfluorododec-6-ene and trans-perfluorododec-5-ene at a rate of 1: 1 and perfluorocyclohexene.
To be continued |
Fluorine Notes, 2008, 60, 3-4