Fluorine Notes, 2008, 60, 5-6
Tripropylammonium and Trimethylammonium fluorotrioxomolybdate (VI) (TriPrAFM) (TriMAFM): Two mild and efficient oxidants for oxidation of organic substratesZ. Javanshira, S. Ghammamyb, K. Mehrania, N. Babayania aDepartment of Chemistry, Faculty of Science, Islamic Azad University, Ardebil Branch,
Ardebil, Iran Abstract Two new mild molybdenum (VI) oxidizing agents: Tripropylammonium fluorotrioxomolybdate (VI)(TriPrAFM) and Trimethylammonium fluorotrioxomolybdate (VI) (TriMAFM) were prepared and characterized. These new compounds are effective in the oxidation of organic substrates specially alcohols to corresponding carbonyl compounds. These compounds were characterized by IR, UV/Visible, 13C-NMR, 1H-NMR and 19F-NMR techniques. The electronic and vibrational spectra of TriPrAFM and TriMAFM have been measured and studied. Keywords: Molybdenum (VI), Tripropylammonium fluorotrioxomolybdate, Trimethylammonium fluorotrioxomolybdate, Oxidation, Organic substrate, Alcohols Introduction In recent years, significant improvements were achieved by the use of new oxidizing agents1-3 such as Pyridinium dichromate (PDC)4, Pyridinium fluorochromate (PFC)5, Triphenylmethylposphonium chlorochromate6, Chromium trioxide-3,5- Dimethylpyrazole complex (CrO3 3,5-DMP)7, Tributylammonium chlorochromate (TriBACC)8, 3,5-Dimethylpyrazolium fluorochromate (DmpzHFC)9, Quinolinium fluorochromate (QFC)10, 2,2'- Bipyridinium chlorochromate (BiPCC)11. Many of above reagent be used the ammonium cation with chromate. Now we want to use ammonium cation with molybdate. In this research Pr3N and Me3N with HF were used to synthesis tripropylammonium fluorotrioxomolybdate (VI) (TriPrAFM) and trimethylammonium fluorotrioxomolybdate (VI), (TriMAFM). These new compounds are efficient and have certain advantages over similar oxidizing agents in terms of the amount of oxidant and solvent required, short reaction times and high yields. RESULTS AND DISCUSSION These compounds can be easily prepared in good yield, quite stable when stored dry and in the absence of light, and are active as oxidizing agents for the conversion of alcohols to carbonyl compounds. It has been found that these reagents have certain advantages over similar oxidizing agents in terms of the amounts of oxidant and solvent required, and especially in the short reaction times required and in the higher yields of the product (Table I and Table II).13-16
Table I. Oxidation of alcohols and polycyclic arenes with TriPrAFM
Table II. Oxidation of alcohols and polycyclic arenes with TriMAFM
In conclusion, the lower acidity of these reagents, the easiness of preparation, theirs stability, non-hygroscopicity, the ease of the work up of the reaction mixture, reasonable yields of products and reaction time make them versatile and practical reagents for the oxidation of alcohols and a useful addition to the presently available reagents in organic synthesis. TriPrAFM and TriMAFM reagents are easy to handle, can be weighed and have no hazardous effects. TriPrAFM and TriMAFM in dichloromethane also oxidize primary and secondary alcohols to, respectively, the corresponding aldehydes or ketones with high yields (Table I and Table II). The results obtained with these reagents are very satisfactory and show the new reagents as a valuable addition to the existing oxidizing agents. Experimental MoO3 (Merck, p.a.) was used without further purification. Solvents were purified by standard methods. Melting points were determined in open capillaries on "Electrothermal 9200" apparatus and are not corrected. Infrared spectra were recorded (KBr disks) on a "Shimadzu" model 420 spectrophotometer. The UV/Visible measurements were made on an Uvicon model 922 spectrometer. 1H-NMR, 13C, 19F NMR were carried out on a Bruker AVANCE DRX 500 spectrometer at 500, 125, 470.66 MHz. All the chemical shifts are quoted in ppm using the high-frequency positive convention. 1H and 13C NMR spectra were referenced to external SiMe4 and 19F NMR spectra to external CFCl3. The percent composition of elements was obtained from the Microanalytical Laboratories, Department of Chemistry, OIRC, Tehran. Preparation of Tripropylammonium fluorotrioxomolybdate (VI), [(C3H7)3NH][MoO3F], TriPrAFM (C3H7)3NH[MoO3F] was prepared by the reaction of (C3H7)3N, MoO3 and HF (1:1:1 ratio) in acetonitrile solvent as follows: (C3H7)3N + HF + MoO3 Molybdenum (VI) oxide (1 g, 7 mmol) was dissolved in dry acetonitrile in a beaker and 30 % hydrofluoric acid (0.1 ml, 7mmol) was added with stirring at 0°C. To the resultant, tripropylamine (0.3 ml, 7 mmol) was added dropwise with stirring to this solution over a period of 0.5 h and stirring was continued for 2h at 0 °C. Within 5 min, a clear white solution formed gave solid TriPrAFM, which was isolated by filtration. The solid was washed with hexane and dried under vacuum for 1 h. In the vibrational spectrum of this compound the known bands of cation and anion
were seen14,15,16such as Preparation of Trimethylammonium fluorotrioxomolybdate (VI), [(CH3)3NH][MoO3F], TriMAFM (CH3)3NH[MoO3F] was prepared by the reaction of (CH3)3N, MoO3 and HF ( 1:1:1 ratio) in acetonitrile solvent as follows: (CH3)3N + HF + MoO3 Molybdenum (VI) oxide (1 g, 7 mmol) was dissolved in acetonitrile in a beaker and 30 % hydrofluoric acid (0.05 ml, 7mmol) was added with stirring at 0 °C. To the resultant solution, trimethylamine (0.3 ml, 7 mmol) was added dropwise with stirring to this solution over a period of 0.5 h and stirring was continued for 2h at 0 °C. Within 5 min, a clear white solution formed gave solid TriMAFM, which was isolated by filtration. The solid was washed with hexane and dried under vacuum for 1 h. In the vibrational spectrum of this compound the known bands of cation and anion
were seen14,15,16such as Oxidation of alcohols: General Method The alcohol (0.001 mol) dissolved in a small amount of the solvent was added to TriPrAFM (0.001 mol) in CH2Cl2 (25 ml) at room temperature. The mixture was stirred and refluxed for the time indicated in the Table 1 at room temperature, diluted with CH2Cl2 and filtered. Evaporation of solvent furnished the product. The molar ratio of substrate to oxidant was 1:1. The solution became homogeneous briefly before the black-brown reduced reagent precipitated. The progress of the reaction was monitored by TLC and UV/Visible spectrophotometery (at 228 nm). Same as above to TriMAFM (0.001 mol) in CH2Cl2 (25 ml) was added the alcohol (0.001 mol) dissolved in a small amount of the solvent at room temperature. The mixture was stirred and refluxed for the time indicated in the Table 1 at room temperature, diluted with CH2Cl2 and filtered. Evaporation of solvent furnished the product. The molar ratio of substrate to oxidant was 1:1. The solution became homogeneous briefly before the black-brown reduced reagent precipitated. The progress of the reaction was monitored by TLC and UV/Visible spectrophotometery (at 228 nm). Acknowledgements The authors would like to thank Dr. Gh. Rezaei Behbahani and Dr. Mahjoub for valuable discussions. References
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fluorine Notes, 2008, 60, 5-6
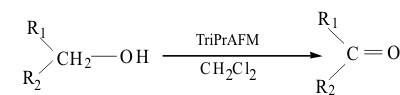
 (C3H7)3NH[MoO3F]
(C3H7)3NH[MoO3F] Mo=O(A1)that
was found at 873.16 cm-1that confirmed with literature
data. The expected signals were found in the1H-NMR and13C-NMR.19F-NMR
shows the bond between Mo and F. IR (KBr): 873cm-1 ν1(A1)
or ν(MoO3), 918cm-1 ν4(E) or ν(MoO3),
598 cm-1 ν2(A1)
or ν(Mo-F). UV/Visible13C-NMR,1H-NMR
and19F-NMR were all consistent with the TriPrAFM structure. Electronic
spectrum of TriPrAFM shows a transition in acetonitrile at 228nm (ε=
355.2 mol.-1lit.cm-1) that belongs to1A1
Mo=O(A1)that
was found at 873.16 cm-1that confirmed with literature
data. The expected signals were found in the1H-NMR and13C-NMR.19F-NMR
shows the bond between Mo and F. IR (KBr): 873cm-1 ν1(A1)
or ν(MoO3), 918cm-1 ν4(E) or ν(MoO3),
598 cm-1 ν2(A1)
or ν(Mo-F). UV/Visible13C-NMR,1H-NMR
and19F-NMR were all consistent with the TriPrAFM structure. Electronic
spectrum of TriPrAFM shows a transition in acetonitrile at 228nm (ε=
355.2 mol.-1lit.cm-1) that belongs to1A1