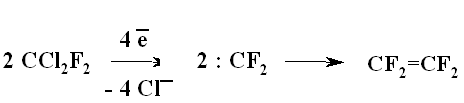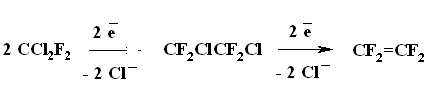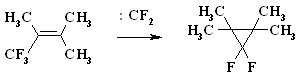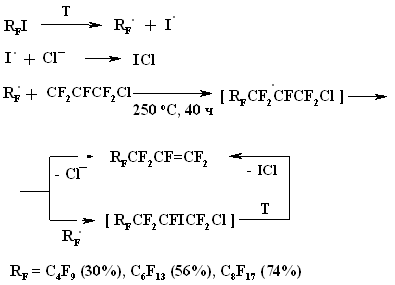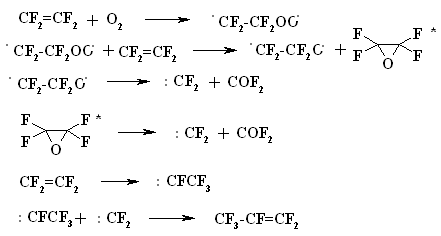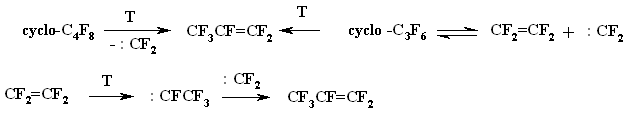Fluorine Notes, 2008, 58, 3-4
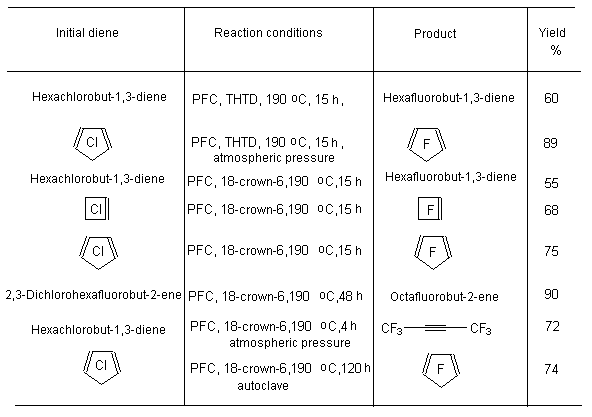
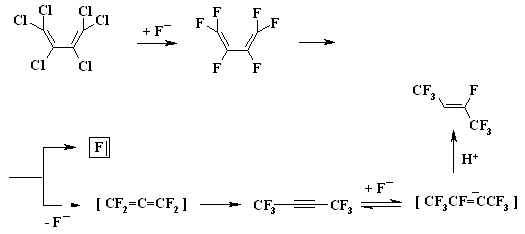
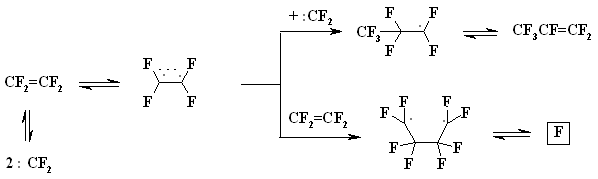
A BREAKTHROUGH IN CHEMICAL TECHNOLOGIES OF FORMING THE MULTIPLE BOND WITH FLUORINE ATOMS AND PERFLUOROALKYL SUBSTITUENTS AT ITA.N. Il'in*, G. G. Furin *614113, Russia, Perm, ul. Lasvinskaya, 98, JSV Halogen (continuation) Carben (:CF2) plays a key role in these processes. It could be supposed, that for other approaches as well, particularly for decomposition of fluorine containing organic compounds and polymers based on them, where difluorocarben is generated, tetrafluoroethylene would be formed. Thus, it is shown at plasmochemical decomposition of fluoroorganic compounds. Although, the forming of tetrafluoroethylrene goes only with the yield of 48% this way may be laid as a basis of alternative commercial method. The attempt to obtain tetrafluoroethylene during the recycling of used polytetrafluoroethylene goods is an important moment for the production of tetrafluoroethylene. Thus, the authors of work [55] offered to conduct the thermolysis of fluoropolymers over an aqueous vapor in a rotating oven at temperature of 500-700 oC in 5-60 sec. This was used to obtain tetrafluoroethylene, hexafluoropropylene and octafluorocyclobutane out of corresponding fluoropolymers. The fluoromonomers obtained are used again for obtaining of fluoropolymeric materials. We should note, that thermal cracking of polytetrafluoroethylene under the special conditions was a subject of studies at the very first stage of obtaining (fluoroplastics) fluorocarbon polymers [56] stillage residues, which are formed during the production of tetrafluoroethylene contain high fluorinated compounds of a wide nomenclature (freon 318C, freon 124, freon 226 and others) [23,57,58]. Part of them is of self-contained interest, for example Freon 318C, other products can become a starting material for the synthesis of new fluoroorganic compounds. Thus, hexafluorochloropropane, which extracting method out of stillage residues was implemented at production site, is a starting material for production of Freon 227ca. Authors of works [59, 60] proved, that during the electrolysis of dichlorodofluoromethane at lead electrode at voltage of 1.5V in acetonitrile tetrafluoroethylene is formed with the yield of 43.2%, which can be easily isolated from other side compounds. It is supposed, that tetrafluoroethylene is obtained by dimerization of difluorocarbene. However, the dimerization of CClF2 radical can't be excluded, it produces 1,2-dichlorotetrafluoroethane. It produces tetrafluoroethylene due to chlorine restoration up to anion at electrolysis. We should note, that difluorocarbene was registered in the reaction with 2,3-dimethyl-2-butene, when 1,1-difluoro-2,2,3,3-tetramethylcyclobutane was isolated as a reaction product. The use of perfluoroalkyliodides is another approach, though they are thermally stable up to 300 oC. Thermal initiation results in their decomposition above that temperature forming perfluoroolefines [61]. Hexafluoropropylene produces perfluoralkenes-1 over a iodotrifluoromethane although the yield is low [62,63]. Telomerization of hexafluoropropylene goes in the medium of perfluoroalkyliodides [61] and α,ω-diiodperfluoroalkanes [64,65]. The authors of work [66] have described an effective obtaining method of terminal perfluorolefines, which lies in heating of pefluoroalkyliodides with perfluoroallylchloride at 180-250oC and atmospheric pressure. We should note, that the rise in temperature up to 200-250 oC doesn't increase the conversion and the presence of CuI or peroxide (Trigonox 145) doesn't lead to the increase of the yield of terminal perfluoroolefin. At that, its isomeriztion into internal perfluoroolefin is not taking place. Terminal perfluorolefines of 99% purity are obtained using this method. The reaction probably goes according to the following scheme: The obtaining method of trifluoroethylene lies in thermal dehydrofluorination of fluorine containing ethanes in the reactor made of chrome and nickel alloy and which inner surface was treated with pyrolysis products over an aqueous vapor at organic/ aqueous vapor proportion equal to 3-2 at temperature of 650-700 oC, after which the pyrolysis is carried out at 700-900 oC [67]. The trifluoroethylene obtaining method based on interaction of CF3CClFX (X = H, Cl, F) with hydrogen over catalysts - Ru, Ni, Cu, Cr metals, halogenides and metals oxides [68]. The exchange of haloids atoms for fluorine at multiple bond in R2CCl=CClR3 (R2, R3 = F, alkyl, fluoroalkyl) halogenated olefin under the influence of alkali metal fluoride, for example fluorolefines like RFC=CFR1 (R and R1 = F, alkyl, fluoroalkyl) in the medium of dimethylformamide or N-methylpyrrolidone [69] can be used to synthesize perfluorinated olefines. 1-3 equivalents of alkali metal fluoride are used for the total amount of chlorine. The authotrs of work [70] proved the efficiency of chlorine exchange for fluorine by potassium fluoride influencing in hexachlorinebuta-1,3-diene, octachlorocyclopentene and hexachlorocyclobutene over perfluoroperhydrophenantrene (PFC) (Table 2). Table 2. Chlorohydrocarbons reactions with KF in perfluoroperhydrophenanthrene medium The following scheme can be supposed for the transformations of hexachlorobut-1,3-diene: 2. Studying of Tetrafluoroethylene Transformation Processes as a Way To Obtain New Semi-Products of Organic Synthesis During the production of tetrafluoroethylene the forming of a number of minor fluorine containing compounds occurs as well as the accumulation of high-temperature bottom product. During the large-scale production of tetrafluoroethylene it is necessary to transform these compounds into useful products. First of all, that goal was connected with the obtaining of hexafluoropropylene as a raw material for the production of a number of fluorine containing materials [21]. The process is a part of fluoropolymers' obtaining technology. High-temperature products formed at pyrolysis of difluorochloromethane after they separated from tetrafluoroethylene represent a mixture consisting of 90.7 % CHF2Cl, 8.25 % CF3CF=CF2 , 0.6 % CF2Cl2 and 0.45 % CF2=CFCl, which is being fractioned with obtaining of 99.6 % CHF2Cl and 99.97 % CF3CF=CF2 [71]. Mechanism and kinetics of the reaction of thermal decomposition of tetrafluoroethylene are studied in details, which to a great extent is caused by the production of hexafluoropropylene [21] based on it. Perfluorocontaining compounds with the number of carbon atoms equal to 4: C4F8-cyclo, perfluorobut-1-ene, perfluorobut-2-tene and perfluoroisobutylene are the main products of tetrafluoroethylene pyrolysis. CF4, C2F6, C3F8, polymerization products and carbon-black (the product of a total destruction) are formed in extremely small quantities (1%). By the increase in temperature of pyrolysis tetrafluoroethylene and hexafluoropropylene (HFP) become the main products. One of the most urgent goals for the production of HFP is a processing of steam-gas mixtures, containing azeotropes, particularly separation from Freon 22. To increase the selectivity of the process regarding hexafluoropropylene at the same conversion of tetrafluoroethylene the increasing of pyrolysis temperature and the decreasing of contact period are needed. Thus, it is proved [72], that during the increase of temperature up to 1123K and decrease of the contact period down to 0.03 - 0.05 sec the selectivity regarding HFP increases up to 70% at conversion of tetrafluoroethylene around 65-70%, which is 20% higher than under the conditions of pyrolysis at 1043K. At increasing of the contact period the yield of HFP goes up. For example, if the contact period is 1 second the yield of HFP will be 45% at the summary yield of tetrafluoroethylene and HFP of more than 70%. The authors of monography [21] recommend the following conditions for the HFP obtaining: temperature equal to 1123K, contact period equal to 1 second, the grade of dilution of the starting material by aqueous vapour within the range of 0.5 - 1.0. They consider a two-step pyrolysis of difluorochloromethane as the most perspective one and not the pyrolysis of high-priced tetrafluoroethylene. At first stage the pyrolysis of difluorochloromethane takes place over the aqueous vapour at increasing of the conversion up to 80-85%, which significantly decreases the amount of returned difluorochloromethane. During the second stage after hydrogen fluoride separating the pyrolysis of difluorochloromethane left is being carried out as well as of a formed tetrafluoroethylene and by-products over the aqueous vapours or not over them. At isolation of HFP using rectification the unreacted tetrafluoroethylene is returning to the second stage of pyrolysis and octafluorocyclobutane (OFCB) is being isolated as a target product or is returning to the second stage of pyrolysis. That approach is based at step-by-step separation of a fraction containing target products, difluorochloromethane, HFP and close boiling admixtures using the method of extra active rectification and adsorption [73,74]. The authors of work [75] described the obtaining method of HFP and OFCB, to which basis the double-stage option is laid. At first stage the thermal decomposition of difluorochloromethane down to tetrafluoroethylene is being carried out. At second stage the dimerization of tetrafluoroethylene with simultaneous forming of HFP and OFCB is taking place by introduction of the last mentioned compound and aqueous vapour into the reactor with fluidised layer of catalyst at temperature of 600-700 oC. The introduction of aqueous vapour through the nozzle allows providing needed temperature control of the process and avoiding forming of solid polymers. It was proved, that at pyrolysis of tetrafluoroethylene at 800 oC and over oxygen gas hexafluoropropylene was formed [76,77]. That observation became a basis for the approach of creating the hexafluoropropylene production technology using tetrafluoroethylene pyrolysis. They have determined the influence of oxygen concentration on the process selectivity according to hexafluoropropylene and on the conversion of tetrafluoroethylene [76]. Based on the experimental data it was proved, that using of oxygen addition in an amount of 1-5 % (vol.) against tetrafluoroethylene allows lifting up the conversion of tetrafluoroethylene by 30% compare to tetrafluoroethylene pyrolysis without oxygen and that selectivity of the process by hexafluoropropylene increases by 20-35% compare to pyrolysis without oxygen. The results obtained conform to the supposed mechanism of thermal decomposition tetrafluoroethylene over the oxygen: The using of oxygen in the amount of less than 1 % (vol.) is insufficient for forming of the necessary amount of (.CF2-CF2OO.) peroxide biradicals; when using the large amount of oxygen (more than 5 % vol.) under the conditions of pyrolysis the destruction of initial tetrafluoroethylene takes place, which is confirmed by the presence of such components as carbon dioxide, hexafluoroethane, carbonylfluoride in the products of reaction [76,77]. The authors of work [78] offered and implemented on the production site an untraditional pollution free hexafluoropropylene concentrating method using membrane method to the concentration higher than azeotrope point, that together with the rectification allows obtaining marketable hexafluoropropylene with purity over 99.9%. Experimental-industrial membrane apparatus is created based on the modified hollow fiber Graviton. It is manageable to reach the level around 12-15% ( mole) of Freon 22 at one-step concentrating in real technological mixtures based on returned Freon 22 , containing 2-6.5% ( mole) of hexafluoropropylene , 3-7% ( mole) (summed up) of trifluorochloroethylene, tetrafluoroethylene and freons 12, 318 and 114. The carrying out of double-stage membrane process allows to obtain the flow containing 45-50% (mole) of hexafluoropropylene. Other perfluorolefines can be obtained out of tetrafluoroethylene. Thus, pyrolysis of a mixture containing tetrafluoroethylene and trifluoromethane (Freon R23) at 700-1000 oC, reagents' contact within the period of 0.01 - 14 sec and trifluoromethane : tetrafluoroethylene ratio as 0.1 : 5 results in forming of hexafluoropropylene of the high yield [79]. Perfluoroisobutylene, CF3CF=CFCF3, C2F3H, CF3CH=CF3, CF3CF2CF=CF2, are formed as admixtures in small amounts, they can be separated using standard methods during the purification of target product. The detailed research of hexafluoropropylene synthesis mechanism at gas-cycle pyrolysis of tetrafluoroethylene included the analysis of intermediate products and kinetic analysis of the alternative ways of synthesis. The combination of experimental and calculation data allowed to create kinetic model of synthesis, where the generating of the most stable difluorocarben in terms of thermodynamic was the key stage. The double bond of fluorolefines can be destroyed much easier than the one of hydrocarbon analogues, that the following data is being demonstrated:
If the forming of perfluorocyclobutane by dimerization of tetrafluoroethylene had been confirmed enough [80,81], then for the long time the ways of forming of hexafluoropropylene during the thermolysis of tetrafluoroethylene might be a subjects of discussion and will be treated in different ways. Thus, the author of work [82] refers that as a fact which occurs due to the thermoisomerization of hexafluorocyclopropane, whilst the authors [83, 84] tend to consider olefine-carbene isomerization of tetrafluoroethylene and the further recombination of carbene :CFCF3 the real ones. The forming of difluorocarbene during gas-cycle pyrolysis (600 - 1200K) of teterafluoroethylene occurs at elementary reaction of biradical structure decomposition. This singlet 1,2-biradicaloid unknown before reacts to difluorocarbene, which later leads to forming of hexafluoropropylene. The building of octafluorocyclobutanic system out of tetrafluoroethylene is going by attaching of that biradical structure to the terafluoroethyelne molecule formig 1,4-biradicaloid with further closing up to four-member cycle [85,86].
To be continued |
Fluorine Notes, 2008, 58, 3-4