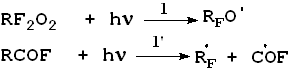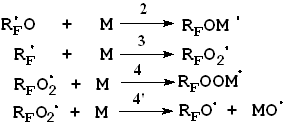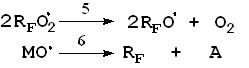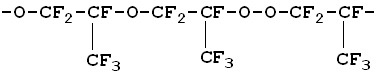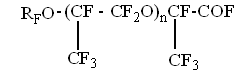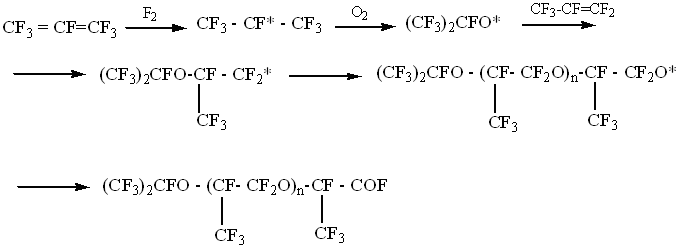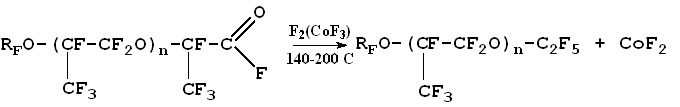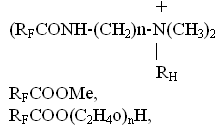Fluorine Notes, 2008, 58, 5-6
Perfluoropolyethers. Synthesis and applicationMaximov B.N., Kornilov V.V., Melnichenko B.A., Kosareva
L.N. Perfluoropolyethers, generally produced through the oxidation of hexafluoropropylene, are fully fluorinated oligomers with alternating fluorocarbon and oxygen links. The general formula of perfluoroethers is as follows:
Those oligomers are known for wide range of their boiling temperatures: from 50-70 oC to 240-320 oC at 1 mm Hg, while their freezing temperatures are about -60 oC or even lower. Perfluoropolyethers possess also a number of very unique properties [1]: high heat resistance, chemical stability in very aggressive media, incombustibility, non-toxicity, excellent dielectric characteristics, viscosity nearly unchanged in wide temperature range, excellent lubricating properties, radioimmunity, etc. The mentioned set of properties makes perfluoropolyethers applicable in various fields of modern industry for liquid dielectrics, heat-carriers, greases and lubricants. Some properties of specific perfluoropolyether fractions are shown in table 1. Table 1. Some properties of perfluoropolyethers
The methods for the manufacture the most popular perfluoropolyethers are all based on hexafluoropropylene (CF3-CF=CF2) that is an industrially available fluoromonomer used for raw material in the manufacture of many fluoroplastics. The current state of perfluoropolyether technology development was preceded by worldwide research undertaken by a number of Russian, USA and Italian companies, the world leaders in this field, in [2-5] in 1970 - 1980ies. They have studied the possibility for perfluoropolyether manufacture starting with all kind of fluoromonomers and using various routs: gas-phase and liquid-phase processes at various temperatures, pressures, etc. The research and development of perfluoropolyether technologies was complicated by the necessity of certain safety conditions observance because oxidation of fluoroolefines by oxygen results in potentially explosive peroxides. Basing both on published data and our own results a process for perfluoropolyethers manufacture is now being developed at RSC "Applied chemistry" using hexafluoropropylene for initial raw material. The process is to be conducted in liquid at low temperature (below -30oC) and under UV-irradiation or under the action of elemental fluorine. The boiling point of hexafluoropropylene (CF3-CF=CF2) is -29.1oC. The density of liquid hexafluoropropylene at different temperatures is shown in table 2. Table 2. Density of hexafluoropropylene at various temperatures
Manufacture of perfluoropolyethers The process for target "neutral" perfluoropolyethers preparation involves the steps as follows:
The analysis has shown that photochemically produced perfluoropolyethers have got "irregular" structure:
and that the low-temperature properties of such perfluoropolyethers exceed those of regular-structured perfluoropolyethers. Study on hexafluoropropylene oxidation in liquid phase Oxidation of hexafluoropropylene was conducted either in quartz or in a metal (stainless
steel) reactor at various temperatures (-20 The most important elemental reactions (5) are as follows:
Here: M = C3F6, RF = CF3, M, A = CF2O, CF3COF Oxidation reactions start under the action of UV emission with wavelength less than 320 nm; low-pressure or medium-pressure mercury lamps are applicable for this purpose (e.g., DRT-400). Hexafluoropropylene, other fluoroolefines and their mixtures with oxygen, when free of impurities and oxidation products, do not absorb UV with wavelength more than 200 nm. That is why the reaction has got a short induction period during which fluoroolefines that are present in negligible amounts convert to some UV-absorbing substances, such as CF3COF or peroxides, and their further photolysis results in peroxide O-O bonds rupture (reaction 1) and/or in acylfluoride C-C bonds rupture (reaction 1') followed by the formation of free radicals that initiate chain reactions between olefins and oxygen (reactions 2 - 4). One cannot also rule out another mechanism of the reaction active centre origination through the formation of donor-acceptor oxygen-hexafluoropropylene complexes with absorption spectra shifted to longer wavelengths. Photolysis of such complexes may result in active species able to initiate oxidation reactions. It is well known that even at low temperature perfluoroalkoxy radicals tend to undergo degradation by C-C bonds, and consequently the radical decomposition (reactions 5, 6) contributes much to the chain development reactions. According to the available test results and published data reactions of peroxyradicals is the most important among all chain interruption processes [6]. Some fluorine-containing peroxyradicals thus formed may undergo recombination (reaction 5) resulting in two perfluoroalkoxyradicals that readily continue the chain. Basing on our mass-spectroscopy results and in conformity with available published data [7] we made the conclusions about the structure of hexafluoropropylene oxidation products: it contains a linear skeleton that consists of perfluoroalkyls C3F6, connected through oxygen atoms or through oxygen-bridge bonds:
The molecules contain two types of terminal groups: alkoxy- (RFO) and fluoroanhydride (-COF) groups. The product molecular weight depends on the hexafluoropropylene oxidation parameters; at -30 — 40oC it is about 3000 - 4000. Polyether peroxides are the mane products of hexafluoropropylene low-temperature liquid-phase photochemical oxidation. The products composition depends on the process conditions, low-molecular gaseous fluorosubstances (COF2, CF3COF, hexafluoropropylene oxide) are also formed though in small amounts. The process parameters dependence of the reaction products yields is shown. Temperature dependence of the rate of hexafluoropropylene oxidation Effective activation energies for fluorosubstances formation within temperature range -45 — +25oC at oxygen pressure 7 bar are shown below in table 4. Table 4. Effective activation energies for fluorosubstances formation
At low temperature (-35 — -5oC) only ~5% of hexafluoropropylene convert to low-molecular compounds: COF2, CF3OF, hexafluoropropylene oxide; while >95% of original hexafluoropropylene turns into oligomer with average molecular weight ~4000. Perfluoropolyether peroxide remains the main reaction product up to -15oC, the yield of low-molecular products (COF2, CF3OF etc.) grows considerably as the temperature exceeds -15oC. Oxygen pressure dependence of the rate of hexafluoropropylene oxidation Our studies on the pressure dependence at -35oC and -15oC within the
pressure range 0 As UV-irradiation intensity grows the oligomer molecular weight goes down to constant value.
The induction period of hexafluoropropylene fluorooxidation by oxygen is negligible.
As the reaction products (perfluoropolyether peroxides) are accumulated initiation accelerates
at the expense of their photolysis. It is well known that the main disadvantages of fluoroolefine
photochemical oxidation are as follows: With allowance maid for the above it seems more promising to apply the method of fluoroolefine/oxygen radical co-polymerization using some chemicals that undergo low-temperature homolytical dissociation e.g., elemental fluorine [6]. According to NMR F19 the structure of thus produced HFP-based perfluoropolyethers is as follows:
here: RF=CF3,(CF3)2CF- It is typical for chemical fluorine-initiated processes that their products in fact do not involve neither fluoroformiate nor difluoromethylene oxide groups those being, however, mandatory in photochemical oxidation products. It is anticipated that chemical fluorine-initiated hexafluoropropylene/oxygen co-polymerization occurs according to as follows:
In the case of chemical fluorine-initiated hexafluoropropylene/oxygen co-polymerization by-production of difluorophosgene (COF2) is rather small (0.01-0.2 mole/mole of perfluoropolyether) that is ten times less than in photochemical process. A peculiarity of the process chemical initiation is the absence of inhibition effects due to the reaction by-products (COF2, CO, CO2, CF3OF etc.). Unlike it, in the case of photochemical hexafluoropropylene/oxygen oxidation by-products completely inhibit co-polymerization process if their concentration in gas phase exceeds 30% by volume. However, chemical initiation of hexafluoropropylene/oxygen co-polymerization requires strict compliance with the process parameters. Fluorine slippage can result in self-ignition and monomer combustion in oxygen medium. In this specific case the choice of the method for perfluoropolyether manufacture was dictated both by the safety considerations and by hardware implementation possibilities for the process of hexafluoropropylene/oxygen co-polymerization. Fluoroanhydride group from perfluorooligoethers produced by hexafluoropropylene/oxygen co-polymerization is eliminated on exposure to elemental fluorine, CoF3 or other fluorinating agent:
The reaction results in neutral perfluoropolyethers. Starting from fluoroanhydrides of perfluoropolyether acids a range of efficient fluorinated surfactants was prepared: cationogenic, anionogenic, non-ionogenic [7]
Those surfactants have found many applications as components of fire-fighting foams (oil products fire extinguishing), oil additives, lubricants, diesel fuels, addition agents decreasing friction and improving performance of various machines, mechanisms and other technical devices. References: 2. D.Sianesi, A.Pasetti. J. Org. Chem, 31, 2312 (1966) 3. D.Sianesi, A.Pasetti. J. Am. Chem. Soc. Polymer. Preprints, 1971. V. 2, N 1, P411. 4. J.A.Hovard, K.Ingold. San. J. Chem, 1965, V. 43, 2729. 5. .T.Heiklen, V.Kaight, J. Phys. Chem, 1965, V. 69, N 7, 2484. 6. N. A. Ryabinin i dr., Patent RU 2046127, 1993. 7. N. A. Ryabinin, B. N. Maksimov, Patent RU 2069673, 1995. |
Fluorine Notes, 2008, 58, 5-6
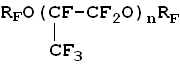
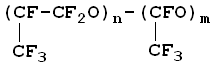
 -40oC). The metal reactor (steel X18H10T) was a cylinder capped with a screw
cap provided with a quartz window, and equipped with a pocket for thermocouple, Gopher
valve, and thermostating jacket. High-pressure mercury-quartz lamp DRT-400 (220 V, AC)
was used for the irradiation source. Hexafluoropropylene from a cylinder passed a column
filled with silica gel and molecular sieves (to remove humidity and other impurities)
and then condensed in a reactor. The reactor was cooled to the predetermined temperature
and oxygen was fed into it under UV-irradiation. On completion of the process we evacuated
all gaseous reaction products, opened the reactor and poured the reaction mass into a
special tank for further stabilization and the target products isolation through vacuum
distillation. Photooxidation is a complicated chain reaction with the participation of
three types of radicals:
-40oC). The metal reactor (steel X18H10T) was a cylinder capped with a screw
cap provided with a quartz window, and equipped with a pocket for thermocouple, Gopher
valve, and thermostating jacket. High-pressure mercury-quartz lamp DRT-400 (220 V, AC)
was used for the irradiation source. Hexafluoropropylene from a cylinder passed a column
filled with silica gel and molecular sieves (to remove humidity and other impurities)
and then condensed in a reactor. The reactor was cooled to the predetermined temperature
and oxygen was fed into it under UV-irradiation. On completion of the process we evacuated
all gaseous reaction products, opened the reactor and poured the reaction mass into a
special tank for further stabilization and the target products isolation through vacuum
distillation. Photooxidation is a complicated chain reaction with the participation of
three types of radicals: