Fluorine Notes, 2008, 57, 3-4
A BREAKTHROUGH IN CHEMICAL TECHNOLOGIES OF FORMING THE MULTIPLE BOND WITH FLUORINE ATOMS AND PERFLUOROALKYL SUBSTITUENTS AT ITA.N. Il'in*, G. G. Furin *614113, Russia, Perm, ul. Lasvinskaya, 98, JSV Halogen (continuation) 1. Improvement of the Obtaining Method for teterafluoroethylene as basic compound for production of different fluorine containing semi-products and materials based on them. Creating of stable tetrafluoroethylene base is the most important condition for functioning of fluoroorganic businesses. Since the 1940-s the main commercial obtaining method of fluorine containing olefins is thermal decomposition of fluorine and fluorine and chlorine containing methanes, ethanes and olefins. Pyrolysis of difluorochloromethane is a key process for the synthesis of teterafluoroethylene applied as a starting material to obtain different fluoroplastics. Numerous studies of this process allowed developing of a stable functioning technology, which was implemented at a commercial scale in many countries. The works that had been done in recent years were mainly seeking more economical and simple conditions for implementation of that thermolysis. The using of catalytic pyrolysis did not allow improving the process technological figures significantly, and catalysts applied got covered with the carbonaceous formations and they were loosing their activity very fast. The improving of teterafluoroethylene obtaining process proved to be unthinkable without carrying out the large-scale studies of fluorine containing compounds decomposition processes. Analysis and results of these studies are listed in the monograph [21] and below we had only presented some key results regarding this process, which had played an important role. Analyzing new approaches and conditions of carrying out the process of dichloromethane thermal decomposition we can see significant simplifications of tetrafluoroethylene production technology [22-29]. They proved to be the most productive ones at introducing the dissolvent, which is an overheated water vapour [21]. For the purpose of obtaining high conversions by raw material and selectivity according to monomer the process is carried out applying a large excess of water vapour to keep the process temperature at high rate. A rather simple reactor in the form of linear tube mounted into the heated tubes' system is used. As the the price of teterafluoroethylene production is defined by the price of initial dichloromethane the main characteristic of the process is the conversion of starting raw material and pyrolysis selectivity by tetrafluoroethylene. Conversion of dichloromehane is defined by the temperature of pyrolysis and reagents' residence time in the reaction zone. Selectivity by tetrafluoroethylene lies in the correspondence of temperature and residence time of reagents in the reaction zone and in partial pressure of difluorochloromethane vapours as well. The most optimal parameters of the present pyrolysis are: temperature (1028 - 1173K), contact period (0.05 - 0.01 c) and pressure 1.7*105 N/m2. The overheated water vapour is inert quite enough towards the reagent and pyrolysis products and has such advantages as ease of condensation and separation from reaction gases. Difluorochloromethane decomposition in the medium of water vapour can be presented by the following reactions:
Optimal operating parameters for tetrafluoroethylene obtaining process using difluorochloromethane with diluting of reaction mixture by water vapour are: temperature 1073 - 1123K, contact period 0.01 - 0.05 c, rate of dilution is 3-4 moles of water per difluorochloromethane mole. Under these technological parameters and displacing mode of operation the conversion of difluorochlomethane is equal 70-80 % and selectivity by teterafluoroethylene - 95 % [30,31]. A more interesting obtaining method of tetrafluoroethylene consists of carrying out the difluorochloromethane pyrolysis under the water vapour and addition of 1,1,2,2-tetrafluorochloroethane [21,30,31]. This method anticipates the separation of hydrogen chloride and obtaining of hydrochloric acid out of it, multi-step rectification separating the fraction of target product and fraction, including difluorochloromethane and hexafluoropropylene. Water absorption is carried out in the apparatus of column type at a counterflow mode. Azeotropic mixtures are isolated in series. In the beginning they isolate the azeotropic mixture of difluorochloromethane and hexafluoropropylene under the pressure within the range of 3-8 bar and with a boiling point within the range of -42 - -38 oC if we convert it into the atmospheric pressure. Afterwards they isolate the azeotropic mixture of 1,1,2,2-tetrafluorochloroethane and octafluorocyclobutane under the pressure ranging within 2-7 bar and boiling point within the range starting from -15 - 0 oC. The process of thermal decomposition of dichloromethane inside the quartz flow reactor in the temperatures range of 923-1073 K under the additives of molecular chlorine gives an opportunity to obtain tetrafluoroethylene with high yield [28]. It was stated, that in all cases the thermal decomposition of difluorochloromethane is carried out by the first order regarding difluorochloromethane. Introduction of chlorine additive into difluorochloromethane, which undergoes thermal decomposition at fresh quartz wall, results in decreasing of activation energy by 29 kJ and increasing the rate of decomposition twice compare to the decomposition process of pure difluorochloromethaneunder the same conditions [22,28]. The results obtained can be interpreted based on the chain-radical decomposition mechanism of difluorochloromethane. At that there is an increased content of difluorochloromethane, difluorodichloromethane and 1,2-dichlorotetrafluoroethane in decomposition products. Tetrafluoroethylene obtained using this method produces a polymerization product of a high yield, which meets all standard requirements. During the work [22] difluorochloromethane pyrolysis was carried out in the solution of pentafluoroethane during 0.1- 60 sec in the temperature range of 500-700 oC under the pressure equal to 10-30 bar inside the metal reactor with adding of anhydrous hydrogen fluoride as catalyst with further distillation of hydrogen fluoride. The conversion of difluorochloromethane amounted to 20%, selectivity of tetrafluoroethylene amounted to 99%. The modern interpretation of ideas concerning thermal difluorochloromethane decomposition is based upon carbenes mechanism [32-35] :
The slowest stage of difluorochloromethane pyrolysis is its thermal dehydrochlorination reaction which should be considered as limiting stage of the process. The second stage is the reaction of recombination of difluorocarbene. It is rather fast and it goes with low activation energy. Full meanings of constants of the reaction rate and conditions of their determination are listed in the monography [21]. The authors of works [36] showed, that difluorochloromethane pyrolysis kinetics does not depend on the type of diluent, particularly the presence of aqueous vapour does not influence the reaction of difluorochloromethane thermal decomposition. However, under the certain conditions during the pyrolysis of difluorochloromethane and at the presence of aqueous vapour, at temperature range of 873 - 993K we can observe the forming of tetrafluoroethylene, carbon oxide, hydrogen chloride and anhydrous hydrogen fluoride according to the scheme:
The direct synthesis of tetrafluoroethylene out of carbon and fluorine within the volume of electrical arc was developed by the researchers of DuPont Company in 1950-60-s. Although, the yield of target product was not high, at the same time different sources of carbon including organic fluorine compounds were used as raw materials. The review of that works is listed in the monography [21]. It was shown, that the process passed through fluorocarboxylic radicals, formed at temperatures within the range of 1800 - 1900 oC, and during the contact period in the reaction zone in the range of 0.0001 - 10 sec. A special reactor was constructed to carry out the process, at the same time a special vacuum equipment was required, that is difficult to solve technically. Nevertheless, the interest for the method haven't disappeared even nowadays. The most acceptable conditions for the process producing 80-90% of tetrafluoroethylene at practically quantitive using of starting materials are as follows: Temperature range starting from 2000 ending 500 oC, contact period equal of 0.01 - 1 s and pressure within 1 - 30 mm Hg. In Table 1 you can find the data regarding use of other physical effects upon the reagents for the tetrafluoroethylene synthesis. Table 1. |
| Influence category |
|
CF2=CF2, % [admixtures] |
Ref. |
|
|---|---|---|---|---|
| High-frequencyplasma |
Initial CF4 + H2 CF3. →: CF2 + F. |
[C3F2H2, C4FH, C4H2, C2F3H] | 37 | |
Turbulent plasma |
Organofluorine compounds : CF2 CF2=CF 2 |
CF4, C2F2, C2F, F |
38 |
|
Plasma or uniflow electric arc |
C 1-10 (mainly C2F5H or CHF3), inert gas (Ar, HF, CO, N2, CF4, CO2) pressure 0.1 – 2 bar, contact time 0.002 – 0.1 c, T- 1727Ê Pyrolysis C2F5H ( 800 oC, Contact time 0.01 c, Pressure 100 KPa), conversion 100 % Pyrolysis CHF3 |
66.9 – 75.8 % [CF4, C2F6, CHF3, C3F6) 82 % [CF4, C2F6] |
39,40 |
|
| Plasma CF4 |
Carbon dust + F2, ratio C:F = 1:2
: CF2
|
41,42 |
||
| CO2-laser |
Pyrolysis CHF3 , Pressure 0.4 - 4000 Pa Hexafluoroethane pyrolysis Pentafluoroethane pyrolysis Two directions of reaction: C2F5H
C2F5H
Radicals photolysis in the field of laser generation Polytetrafluoroethylene thermolysis Thermolysis C6F6, C6F5H CF... ,:CF2 (249 nm) Perfluorocyclohexane Decafluorocyclopentane Perfluoroethers CH4 + SF6 |
C2F4, CF3CF=CF2, Octafluorocyclobutane 64 80 [CF3CF2O, CF2O] [CS2, CF4 ] |
43,44 45 46-48 49,50 39 51 52 53 54 |
Fluorine Notes, 2008, 57, 3-4
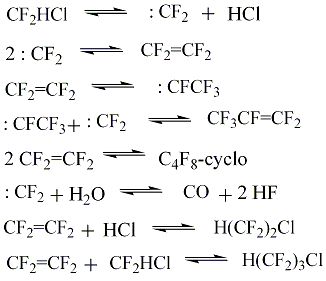
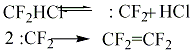
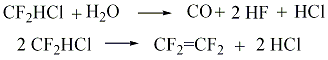
 CF2=CF2
CF2=CF2