Two new Fluoro complexes: Tetramethylammonium trifluoroplumbate (TMATriFP)
and Tripropylammonium tetrafluoroantimonate(TriPATFA)Zahra Javanshira, Shahriare Ghammami a,b*, Kheyrollah Mehrani a aDepartments of Chemistry, Faculty of Science, Islamic Azad University, Ardebil Branch, Ardabil, Iran bDepartment of Chemistry, Faculty of Science, Imam Khomeini International University, Ghazvin, Iran, E-mail: shghamami@ikiu.ac.ir or shghamamy@yahoo.com Abstract Two new complexes synthesized by tetralkylammonium fluoride salts. Tetramethylammonium trifluoroplumbate and tripropylammonium tetrafluoroantimonate have been prepared from N(CH3)4F and PbF2or N(C3H7)3, HF and SbF3using either CH3CN, or excess PbF2or SbF3 as a solvents. The structure of the PbF3-and SbF4- anion was studied by 19F NMR spectroscopy, IR, UV/Visible spectroscopy. Introduction The number of Antimony (III) and Lead (II) fluorocomplexes are still scarce, and very few studies on their reactivity have been reported. Particularly the fluorides have been subject of an intense scientific discussion since the first synthesis of such a compound [1, 2].This is because of the important prerequisites for a fluorinating agents to be useful which are theirs mildness, versatility, selectivity and operational simplicity. The subject of this investigation is obtaining of inorganic fluorides and complexes [3]. Lead and Antimony fluorocomplexes have more attraction because these compounds could be prepared by reaction of fluorinating agents such as CsF, NaF, HF with a complexes. For this purpose we used a new and strong fluorinating agent that is tetramethylammonium fluoride produced at 1990 in anhydrous form [4]. Many fluorocompounds of main group elements were produced using this reagent, namely: PF4- [5], SeF5-, TeF5-, [6] IF8-[7], and in few amounts row of transition metals fluorocomplexes like (CH3)4NMoO3F [8],(CH3)4NCrO3F [9], MoF7-, WF7-, ReOF6- [10], MoO3F- [11]. There were two primary incentives for selection of (CH3)4N+and(C3H7)3NH with HFas the counter ion. Firstly, quaternary ions such as tetramethylammonium and tripropylammonium are often used as phase transfer catalysts. Secondly, quaternary ions such as tetramethylammonium and tripropylammonium are used as crystal growing agents. In this article a direct, simple one-step method has been used to obtaining these compounds. Another reason that encouraged researchers for synthesizing this range of fluorinated compounds is the fewer and rare amounts of the spectroscopic data of this compounds, especially 19 F-NMR data. Some of data were reported in [12-15]. Results and discussion Tetramethylammonium fluoride is an ionic compound and many efforts have been made for preparation of its anhydrous form. It produces by reaction of tetramethylammonium hydroxide with hydrofluoric acid as follow: 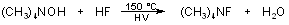
Reagent grade PbF3 was used. Anhydrous tetramethylammonium fluoride (CH3)4NF was prepared by the method reported by Christe. [20] Tetramethylammonium trifluroplumbate (III) (CH3)4N][PbF3], TMATriFPb (CH3)4N[PbF3] was prepared by the reaction of (CH3)4NF and PbF3 in a 1:1 ratio in MeCN solvent as follows: PbF2 + (CH3)4NF  (CH3)4N[PbF3] (CH3)4N[PbF3] The reactant solution color was bright green, and the product color was light green, The typical absorption lines in IR spectra at 469 (s) cm-1 have been attributed to  Pb-F. Absorption lines at 1472(ms), 3095(sh), 499(w, br) cm-1, have been attributed to Pb-F. Absorption lines at 1472(ms), 3095(sh), 499(w, br) cm-1, have been attributed to 15 N-C, 15 N-C,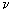 CH3C-H and CH3C-H and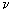 19 N-C of (CH3)4N+ counter ion respectively (Table 1). 19 N-C of (CH3)4N+ counter ion respectively (Table 1). There are two absorption lines in the compound electronic spectra. Electronic spectra of TMATriFPb shows two shifts for Lead 232 nm (  = 280 mol.-1lit. cm-1) and 259 nm ( = 280 mol.-1lit. cm-1) and 259 nm ( = 145 mol.-1lit.cm-1) and that belongs to E = 145 mol.-1lit.cm-1) and that belongs to E E and E E and E A1 shifts (Table 3). These shifts are expected in mono substituted Plumbate ions, because of Lead position making of strong crystalline field complexes. A1 shifts (Table 3). These shifts are expected in mono substituted Plumbate ions, because of Lead position making of strong crystalline field complexes. The expected signals were found in the 1H-NMR and 13C-NMR. Tripropylammonium tetrafluroantimonate (III) (C3H7)3NH [SbF4], TriPATF The inner mobility of fluorine ions and electrophysical characteristics of complex fluorides NaSbF4, KSbF4, RbSbF4, CsSbF4 and SbF3 are studied by means of 19F NMR method and impedance spectroscopy. Types of ion motions in the fluorine subsystem of these compounds in the range of temperatures 180 - 510K are determined.Discrete mononuclear units have been characterized in the form of the matrix-isolated SbF3 molecule and of the anion SbF4–, identified for the first time, in association with appropriately bulky cations in the solid phase and in organic solvents solution; the vibrational spectra of the SbF4– anion imply C2 symmetry. (C3H7)3NH[SbF4] was prepared by the reaction of (C3H7)3N and SbF3 and HF in a 1:1:1 ratio in MeCN solvent as follows: symmetry. (C3H7)3NH[SbF4] was prepared by the reaction of (C3H7)3N and SbF3 and HF in a 1:1:1 ratio in MeCN solvent as follows: SbF3 + (C3H7)3N + HF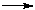 (C3H7)3NH[SbF4] (C3H7)3NH[SbF4] The method for the reaction of (C3H7)3N with SbF3 and HF is as for to (CH3)4NF and its analytical and spectroscopic data are: The reactant solution color was pale white, and the product color was white, IR spectrum at 520 (W, br) cm-1 have been attributed to  Sb-F and absorptions at 1443(m), 3065(sh), 1100(w) cm-1, have been attributed to Sb-F and absorptions at 1443(m), 3065(sh), 1100(w) cm-1, have been attributed to 15 N-C, 15 N-C, CH2C-H and CH2C-H and CNof (C3H7)3N+ counter ion respectively (Table 2). There are one absorption bands in this compound electronic spectrum Electronic spectrum of TriPATFA shows one transitions for Antimony at 271nm ( CNof (C3H7)3N+ counter ion respectively (Table 2). There are one absorption bands in this compound electronic spectrum Electronic spectrum of TriPATFA shows one transitions for Antimony at 271nm (  = 244 mol.-1lit.cm-1) that belongs to B1 = 244 mol.-1lit.cm-1) that belongs to B1 A1 shift (Table 4). The expected signals were found in the19F-NMR, 1H-NMR and 13C-NMR. A1 shift (Table 4). The expected signals were found in the19F-NMR, 1H-NMR and 13C-NMR. Experimental Material and instruments Acetonitrile (Fluka, P.A.) was distilled and dried by phosphorus pentaoxide before using, thereby reducing its water content to <4 ppm. Tetramethylammonium fluoride or Tripropylammonium and fluoric acid were bought from Merck. PbF2and SbF3 (Merck, p.a.) were used without further purification. Solvents were purified by standard methods. Infrared spectra were recorded as KBr disks on a Shimadzu model 420 spectrophotometer. The UV/Visible measurements were made on an Uvicon model 922 spectrometer. 1H and 13C-NMR were recorded on a Bruker AVANCE DRX 500 spectrometer at 500 and 125 MHz, respectively. All the chemical shifts are quoted in ppm using the high-frequency positive convention; 1H and 13C-nmr spectra were referenced to external SiMe4. Antimony and Lead were estimated iodometrically. The percent compositions of elements were obtained from the Microanalytical Laboratories, Department of Chemistry, OIRC, Tehran. Synthesis of Tetramethylammonium trifluoroplumbate (II),[(CH3)4N][PbF3] Tetramethylammonium trifluoroplumbate (II), [(CH3)4N][PbF3] was prepared by dissolving PbF2 (07.1 mg, 2.9 mmol) in MeCN and adding this solution to a solution of tetramethylammonium fluoride (0.29 g, 3.1 mmol) in MeCN under stirring at room temperature until a white precipitate was formed. After 2 hours stirring, the mixture was filtered, washed with ether and dried at room temperature. The tetraethylammonium salts are some what hygroscopic, and it was better to be stored under a layer of hexane, whereas all of the salts are photosensitive and moisture-sensitive, both in solution and solids. Mp: 196oC. C4H12F3NPb: Cacl. %C, 14.19; %H, 3.54; %N, 4.13. Found: %C, 15.30; %H, 3.56; %N, 4.15. UV/Visible, IR, 1H-NMR and 13C-NMR were all consistent with the TMATriFPb structure. Synthesis of Tripropylammonium tetrafluoroantimonate (III), (C3H7)3NH[SbF4] Tripropylammonium tetrafluroantimonate (III), [(C3H7)3NH][SbF4] was prepared as follow: SbF3 (8.9 mg, 5 mmol)was dissolved inMeCN in a beaker and tripropylamine (1.36 ml, 10 mmol) was added dropwise under stirring over a period of 0.5 h and the stirring was continued for 0.5 h at 0 °C. To the resultant clear white solution, hydrofluoric acid 40% (0.2 ml, 5 mmol) was added under stirring at 0 °C. The precipitated white solid was isolated by filtration, washed with hexane and dried under vacuum for 2 h at room temperature. Mp: 275°C. C9H22F4NSb: Calc. %C, 31.60; %H, 6.43; %N, 4.09. Found: %C, 33.15; %H, 6.45; %N, 4.11. UV/Visible, IR, 1H-NMR and 13C-NMR were all consistent with the TriPATFA structure. Conclusion Two tetramethylammonium fluoride and tripropylammonium fluoride salts of SbF3were synthesized simply. (CH3)4N[PbF3] was prepared by the reaction of (CH3)4NF and PbF2 in a 1:1 ratio in MeCN solvent and (C3H7)3NH[SbF4] was prepared by the reaction of (C3H7)3N, HF and SbF3 in a 1:1:1 ratio in MeCN solvent. Electronic and vibrational spectra of these two new Fluoro-complexes were studied. These compounds were characterized by IR, UV/Visible, and 13C-NMR and 1H-NMR techniques. Production of these compounds shows the ability of tetramethylammonium fluoride and tripropylammonium With fluoric acid in fluoride addition to main group elements compounds. References [1]. N. Bartlett, Proc. Chem. Soc. 1, 218,1962. [2]. M. Lein, G. Frenking, J. Chem. 57, 1191, 2004. [3]. E. L. Muetterties, j. Am. Chem. Soc.81,1959, 1084. [4]. K. O. Christe, W. W. Wilson R. D. Bau, J. Am chem. Soc. 112, 7619,1990. [5]. K. O. Christe, D. A. Dixon, H. P. A. Mercier, J. C. P. Slnder, G. J. Schrobilgelq, W.W. Wilson, J. Am. Chem. SOC.116,2850,1994. [6]. A. R. Mahjoub, D. Leopold, K. Seppelt Z. anorg. Chem. 83, 618,1992. [7]. A. R. Mahjoub, K. Seppelt Angew. Chem.Int. Ed. Engl. 30, 876, 1991. [8]. A. R. Mahjoub, S. Ghammami, A. R. Abbasi, A. Hosseinian, J. Chem.Research, 486. [9]. A. R. Mahjoub, S. Ghammami, A. R. Abbasi, A. Hosseinian, Indian J. Chem.39A, 434,2000. [10]. S. Giese, K. Seppelt, Angew. Chem. Int. Ed. Engl. 33, No. 4, 461, 1994. [11]. A. R. Mahjoub, S. Ghammami, A. R. Abbasi, A. Hosseinian, J. Chem.Research, 486. [12]. A. Lehtonen, R. Sillanpaa, Polyhedron. 13, 2519, 1994. [13]. R. H. Grubbs, Comprehensive Organometalic Chemistry. 8, 1193, 1982. New York. [14]. S. Berger, S. Braun, H-O. Kalinowski, "NMR Spectroscopy of the Non-metallic Elements " 1997, John Wiley & Sons Ltd. [15]. R. R. Schrock, Reaction of Coordinated Ligands, Plenum Press, New York, 1986. Table1. The frequencies (cm-1) and assignment of cation and anion of (CH3)4N[PbF3] 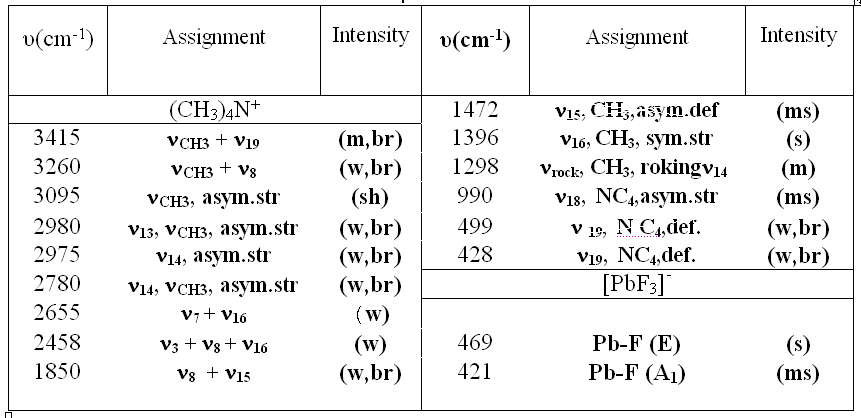
Table2. The frequencies (cm-1) and assignment of cation and anion of (C3H7)3NH[SbF4] 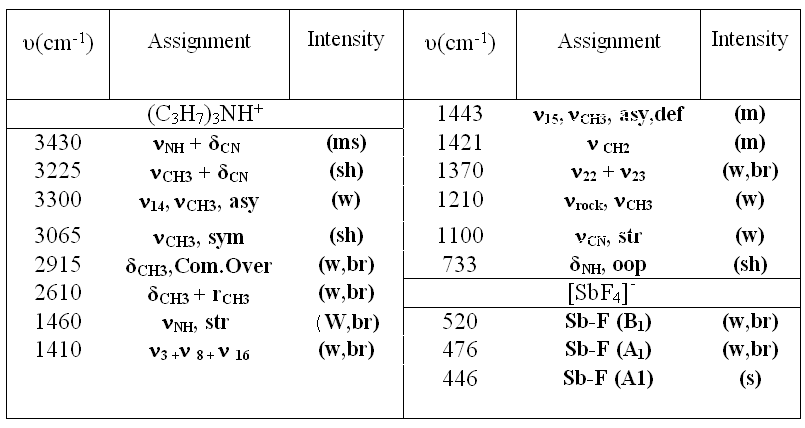
Table 3. Transitions specifications of TMATriFPb 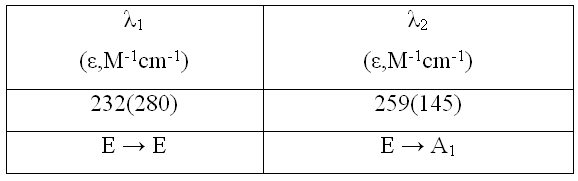
Table 4. Transitions specifications of TriPATFA 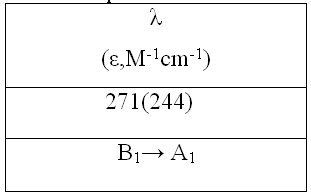
|