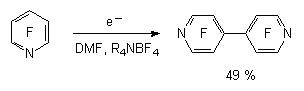Fluorine Notes, 2007, 54, 3-4
The Synthesis of Low-Fluorinated Compounds Based on Polyfluoroaromatic CompoundsG. G. Furin Siberian Branch of Russian Academy of Sciences Novosibirsk N.N. Vorozhtsov Institute of Organic Chemistry of SB RAS Abstract The analysis of obtaining methods for low-fluorinated derivatives of aromatic row including processes of transformation of polyfluoroaromatic compounds had been carried out: using reducing hydrogenolysis of C-F, C-Cl bonds , electrochemical transformation and protodehalogenation by the influence of zinc, copper, nickel in different media. Here the examples on using that processes with the yield of significant semi-products and materials are given. Table of Contents 2. Approaches to Synthesis of Partly Fluorinated
Benzene Derivatives 3. Protodehalogenation of Polyfluorinated Benzene Derivatives Using Metals
2. Approaches to Synthesis of Partly Fluorinated Benzene Derivatives |
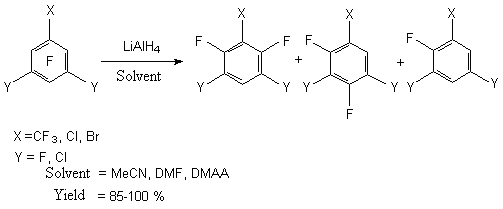
The reduction of chlorine and fluorine containing arenes allows getting closer to the precious incompletely fluorinated benzenes, which are semi-products for the synthesis of the whole range of essential medicals and materials for agriculture. 2.1. Reductive Dehalogenation of Polyfluorochloroarenes by Lithium Aluminium Hydride We can name two processes as synthesis methods for partly fluorinated benzenes among the approaches, resulting in exchange of fluorine for hydrogen in polyfluoroarenes. One of which is based practically on nucleophilic substitution of fluorine directly for hydrogen under the influence of LiAlH4 [4,9,12-15]. In this case, as a rule, the fluorine atom is affected, which is in para-position to the substituent in benzene ring.
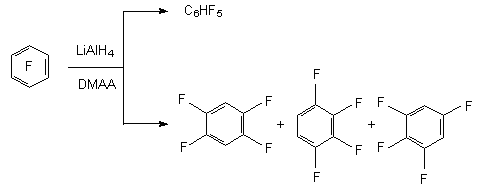
The application of bipolar aprotonic solvent like N,N,-dimethylacetamide results in substitution of several fluorine atoms in hexafluorobenzene at reducing of LiAlH4 [13]. For example, during the reaction of hexafluorobenzene and sodium borane in N,N- dimethylacetamide isomeric tetrafluorobenzenes are formed along with pentafluorobenzene [9].
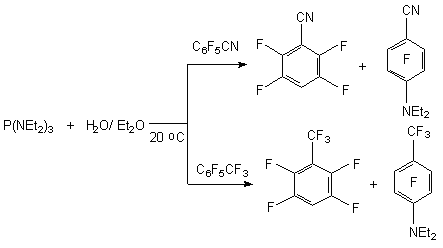
The reduction of polyhalogentoluols and benzenes by sodium borane in polar solvents results in forming of a mixture of products with the common yield equal to 90% with the predominance of para-isomer [9]. The rate of products depends on the selection of the solvent and reaction conditions. 2.2. Catalytic Hydration of Polyfluoroaromatic Compounds Catalytic hydration of polyfluoroaromatic compounds using hydrogen over the noble metals, for example palladium, is one of the variants for obtaining of partly fluorinated benzenes [16,17]. However, the hydration of hexafluorobenzene over the palladium coated coal at 300 oC goes without any selection forming a mixture of products containing different number of fluorine atoms [18]. Especially this process works very well during the substitution of haloides different from fluorine atom. Thus, hydrodechlorination is carried out when palladium influences 2-chlorine-3,5-difluoroaniline at coal over triethylamine [19], 3-chlorine-a,a,a,4,5-pentafluorotoluole in methyl alcohol [20] and 2,3-difluorochlorobenzene in ethyleneglycole [21]. If there is a nitro-group in benzene ring it will become possible to substitute two chlorine atoms for hygrogen. Thus, during the reduction of 2,6- dibrome [22] and 2,6-dichloro-3,5-difluoronitrobenzenes [22, 23] using hydrogen over palladium and over the base not only do we have the substitution of haloid for hydrogen but we get the reduction of nitro-group as well. 3,5-dichlorotrifluorobenzoic acid is introduced into the process; the following conditions are being applied: the solvent- the alcohol at 40 oC [24], 3,5-dichlorotrifluorobenzonitrile in acetonitrile over the natrium carbonate at 82 oC [25] and dichloro-2,4,6-trifluorobenzene in aqueous NaOH at 100 oC [26]. At that the two chlorine atoms have been exchanged for hydrogen at the total yield of final products no less than 80 %. In the work [27] the information on hydration of mixture containing a- and b-chloroheptafluoronaphtalenes above palladium is given, in this case the mixture containing 30% of a-heptafluoronaphtalene and 70% of b-heptafluoronaphtalene was obtained with the yield of 80%. Under the same conditions the hydrodechlorination of pentafluorochlorobenzene was carried out till the obtaining of pentafluorobenzene and the same process was accomplished with the mixture of tetradichlorobenzenes till the obtaining of isomeric tetrafluorobenzenes. Thus, the catalytic hydration of polyfluorochlorobenzenes can be a rather convenient obtaining method of partly fluorinated fluorobenzenes, it can be implemented at a commercial scale. 2.3. Reductive Dehalogenation of Polyfluoroarenes by P(NEt2)3 in Aqueous Media The reactions of polyfluoroarenes selective hydrodehalogenation are of synthetic interest for obtaining of partly fluorinated benzene derivatives. One of the new approaches to carrying out of this process is using of hexaethylphosphotriamide in aqueous organic solvents. It is stated [28], that under the influence of P(NEt2)3 on polyfluoroaromatic compounds over the water the substitution of fluorine atom for hydrogen takes place with the orientation corresponding to one at nucleophilic substitution. For example, in aqueous diethyl ester the influence of P(NEt2)3 on pentafluoropyridine results in forming of 2,3,5,6-tetrafluoropyridine and 4-diethylamino-2,3,5,6-tetrafluoropyridine. The use of more polar solvents for example dimethylformamide gives us exclusively 4-diethylamino-2,3,5,6-tetrafluoropyridine [28,29].
For pentafluorosubstituted benzenes derivatives containing electron seeking groups the following picture is observed - along with product of substitution of fluorine atom, which is in para-position to substituent we get the forming of product of substitution of fluorine atom for diethylamino-group as well. At that by the increasing of temperature and transforming into more polar solvent the share of fluorine substitution for diethylamino-group increases [29].
Pentafluorochlorobenzene remains constant at room temperature for 5 days at holding with aqueous P(NEt2)3, while the bromopentafluorobenzene and iodopentafluorobenzene are reduced under these conditions in a few minutes [30]. Instead of water methyl alcohol can be used. Thus, pentafluorobenzene is formed with a quantitive yield out of bromopentafluorobenzene at room temperature in 5-10 minutes.
The presence of both electrone-acceptor and electrone-donor substituents in benzene ring under the influence of P(NEt2)3 in aqueous organic solvents does not tell upon the character of the products formed, influencing only the target product yield. At that only the bromine atom is being substituted.
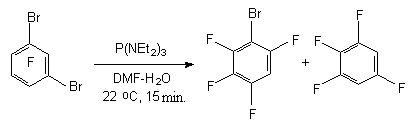
If in the molecule of perfluoroarene there is more than one bromine atom then the process of their successive substitution for the hydrogen atom is possible. Thus, when the equivalent of P(NEt2)3 influences 1,3-dibromotetrafluorobenzene the mixture of 1-bromo-2,4,5,6-terafluorobenzene and 1,3,4,5-tetrafluorobenzene is formed while in the excess of P(NEt2)3 the both bromine atoms are being substituted for hydrogen.
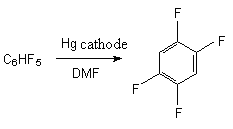
2.4. Electrochemical Reduction of Fluorine Containing Benzene Derivatives. Selective reduction using electrochemical method is an important component of the obtaining method for partly fluorinated aromatic compounds. The using of mercury electrode for these purposes allows the reducing of pentafluorobenzene, 1,2,3,5-tetrafluorobenzene, 1,2,4,5-tetrafluorobenzene, 1,3-difluorobenzene and also 4,4'-difluorodiphenyl and decafluorodiphenyl [31, 32] in the solution of dimethyl-formamide. At that, the fluorine atom is affected, which is in para-position to the hydrogen cycle and partly fluorinated benzene derivatives are formed. Here we should notice that the total reduction of benzene fluorine derivatives is possible. Thus, meta-difluorobenzene [33] and hexafluorobenzene [34] are reduced to benzene.
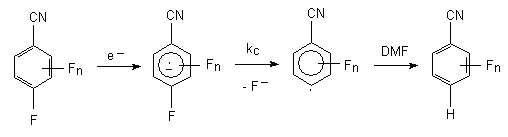
The electrochemical reduction of fluorine containing benzene derivatives stands at a very important place, what is caused by the intensive study of the electron structure and reactivity of anion-radicals of halogen-aromatic compounds [35]. In the framework of this project we have carried out the reduction of fluorinated benzonitriles in dimethylformamide and we have obtained very good resolution spectra of electron paramagnetic resonance (EPR). It is supposed, that the reduction process goes through the intermediate generation of anion-radical of benzene derivative, which during the reaction process is being decomposed forming the aryl radical and ion fluoride. Such anion-radicals were settled by EPR method for the compounds, containing electron acceptor substituents in benzene ring. In case of fluorine containing benzonitriles the reduction leads to the forming of products of fluorine atom substitution, which is in para-position to CN-group, for hydrogen atom [36-38].
As it is stated in the works [39,40] the constant of anion-radicals fragmentation rate depends on the number and disposition of the fluorine atoms in benzene ring.
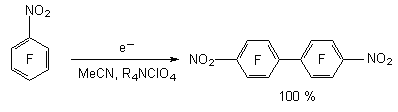
This data shows us the increasing of fragmentation rate by the increasing of the fluorine atoms number in benzene ring. In this row we also observe the increasing of anion-radicals stability by the increasing of fluorine atoms number in benzene ring for the corresponding nitrobenzenes [41, 42]. However, it should be noted, that during the electrochemical reduction of pentafluoronitrobenzene, for example in acetonitrile, dimethylformamide and at the absence of proton donor in system in the generated intermediate anion-radical we do not see the substitution of fluorine atom for hydrogen and we see forming of 4,4'-dinitrooctafluorodiphenyl with practically quantitive yield [43].
The situation like that is observed as well at the electrochemical reduction of pentafluorpyridine [44] and at the same time the presence of a rather strong proton donor in the system, which hydroquinone is ,the product of substitution of fluorine atom for hydrogen in
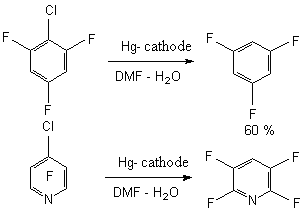
Regioselective reductive dehalogenation at electrochemical reduction of polyfluoro- and polyfluorochloroaromatic compounds was studied in details [41-47]. The authors connect the easiness of reduction with the size of electrochemical reduction potential [32,36-48]. When using aqueous solutions of dimethylformamide we can manage to carry out the hydrodechlorination. Thus, we have obtained 1,3,5-trifluorobenzene [47] out of 1-chlorine-2,4,6-trifluorobenzene, 1,3,5-trifluorobenzene was isolated out of 1,3,5-trifluorotrichlorobenzene with the good yield, and 2,3,5,6-tetrafluoropyridine [45] was isolated out of 4-chlorotetrafluoropyridine [45].
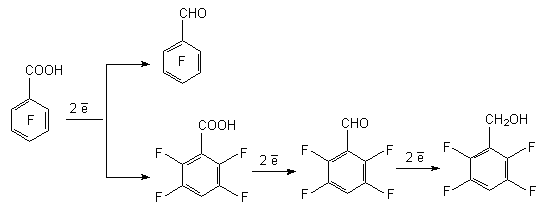
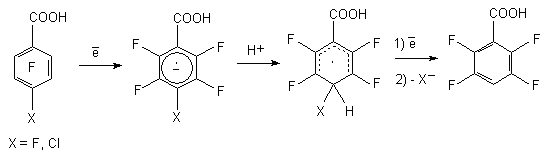
Electrochemical reduction of pentafluorobenzoic acid is carried out at mercury or leaden cathode in aqueous solutions of acids over Et4NBF4 electrolyte and several products are obtained, that is pointing out the simultaneously passing processes of hydrogenolysis of C-F bond and reduction of COOH group [46]. At that the hydrogen atom is affected, which is situated in para-position towards COOH group. The reduction of pentafluorobenzoic acid in 50% sulphuric acid at electrode potential equal to -1.20 V results in forming of 2,3,5,6-tetrafluorobenzoic acid, which concentration in the reaction mixture is 67%, while in 0.2M solution of Et4NBF4 at the potential equal to -2.00 V the total transformation of pentafluorobenzoic acid forming 2,3,5,6-tetrafluorobenzoic acid and 2,3,5,6-tetrafluorobenzyl alcohol (yield equals to 40 and 55% respectively) takes place.
The electrochemical reduction of pentafluorobenzaldehyde, pentafluorobenzoic and 4-chlorotetrafluorobenzoic acid amides take place similarly. The most probable mechanism of these process include initial formation of pentafluorobenzoic acid anion-radical [46].
to be continued |
Fluorine Notes, 2007, 54, 3-4
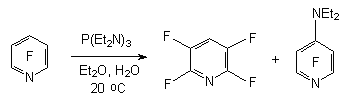
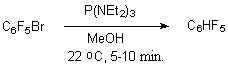
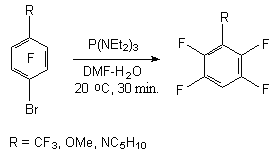
 -position of pyridine ring is formed [45].
-position of pyridine ring is formed [45].