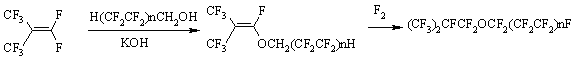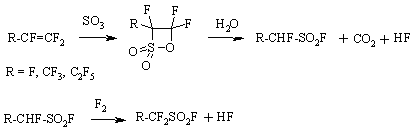Fluorine Notes, 2007, 52, 3-4
ADDITIONAL FLUORINATION OF PARTLY FLUORINATED ORGANIC COMPOUNDS BY ELEMENTAL FLUORINE AS AN OBTAINING METHOD FOR PRACTICAL PURPOSE FLUORINE MATERIALSD.D. Moldavsky, G.G. Furin*
(continuation) 2. Perfluorinated Organic Compounds Purification Data listed above allowed us to draw an assumption, that such an approach can be successfully used for deep purification of perfluorinated compounds ("crude materials") obtained using ECF method or CoF3 fluorination method. All known methods are based on destruction of hydrogen containing admixtures. If we take into account that their content in the reaction mixtures reaches 30%, then these methods can't be recognized as wholly satisfactory. Another approach for purification of PFOC (perfluorinated organic compounds) is in additional fluorination of hydrogen containing admixtures. This approach in case of its implementation allows increasing of yield of PFOC, lowering of waste products, simplifying the rectification and as a result decreasing the cost of target products. The known method of purification is in high concentration aqua alkali (85-90%) treatment at 150 - 200 oC. This method has a number of essential disadvantages, which hampers its application in production quantities, among them are: a need of working under pressure of 13-15 bar, high melting point of high concentrated alkali and a related to that danger of blocking armature and communications, and also a necessity to use special alloys for reactor making, as common stainless steel are up to corrosive cracking [58]. It was found [59], that water displacement by more major solvents (amines or alcohols) allows decreasing of admixtures decomposition temperature to 60-80 oC and working without excessive pressure. We used alcohol-water solutions of KOH, which dissolve forming KF well, for our work. The process was carried out in the apparatus equipped with stirrer, dosing the crude material into aqua-alcohol alkali (1:1:1) accompanied by 14 hour ageing at 60-80oC and further rectification. Products, obtained using such method are characterized as "technical" and their warranty storage period is 6 years. Due to simplicity of apparatus, the method has been implemented commercially, though it is characterized by large amount of waste. Polyfluorinated compounds practically do not react with gaseous fluorine under 150oC. Above this point we can observe burning, and sometimes explosions. Low molecular polyfluorinated paraffins and soot are the main products of this reaction. Fluorinating using catalysts that is iron, cobalt, copper, manganese and nickel fluorides, proved to be more successful. At Fig.2 we can find the information on catalytic fluorination of the "crude material", obtained by electrochemical fluorination of tributylamine. Fig.2. Catalytic Fluorination of Hydrogen Containing Admixtures of the Perfluorotributylamine
"Crude material" (Temperature 420 oC, contact period 13 min., A -
amount of ion-fluoride bound (mole
* l-1),
We can see, that fluorination is going in a time gap, at that depending on the nature of catalyst the fluorination goes with different depth (it was controlled according to the amount of ion-fluoride bound). Hydrogen containing admixtures fluorination goes at a most full grade when using nickel difluoride. However, its activity drops sharply in a few hours time. Cobalt trifluoride properties are close to nickel difluoride, its catalytic activity lasts much longer and is in between the lap of 200 and 350 hours. In Table 10 one can see the results of fluorination using CoF3 of some of the "crude material", obtained at electrochemical fluorination. Table 10. Parameters of Different "Crude Material" Elemental Fluorine Fluorination Processes [5]
According to this method we managed to obtain samples of perfluorinated compounds (eliminated ion-fluoride equal to (1-3) * 10-5 mole/l) which are not absorbed in the area of 200 - 320 nm. Such samples can be qualified as "pure", their guaranteed shelf life is 20 years and they can be used in techniques for creating of medical preparations and materials. In Table 11 one can find the characteristic of commercially produced perfluorinated compounds, obtained by additional fluorination of "Crude Material" using elemental fluorine direct fluorination using cobalt trifluoride as catalyst. Table 11. Perfluorinated Dielectrics-Heat-Transfers (PFDT) Developed at Federal State Unitary Enterprise RSC "Applied Chemistry"
In our opinion, the major advantage of this approach is the fact, that admixtures do not separate from the main product, and on the contrary, they transform into useful perfluorinated components, which not only do not decrease the quality' target product, but also become suitable for use of composition mixtures of perfluorinated compounds, they also significantly increase the efficiency of fluorine use. The decrease of waste, which utilization is a very challenging and complicated task, is very important as well. At that, it is becoming possible to replace the rectification with distillation, that also simplifies the technology and reduces the cost of it. 3. Fluorine Containing Semi-Products Synthesis fro Creating of New Generation of Electrolytes and Dielectrics The semi-products of fluororganic synthesis are of interest of production of new materials. Sometimes, such products themselves can find their application, but the base consists of fully fluorinated compounds. Here we shall draw a purification of emissions of plants producing fluororganic compounds products, which as a rule contain high toxic agents, for example, perfluoroisobutylene, as an example. At this drawing we can see its transformation into effective liquid dielectric by additional fluorination using elemental fluorine. Additional fluorination can be carried out using electrochemical fluorination
method for compounds like The second example refers to obtaining of perfluoroalkansulfofluoride, a key semi-product for obtaining of electrolytes based on lithium bis(perfluoroalkylsulfonylfluoride)imide salt. Here we used obtaining of sultone, cycle opening, decarboxylation and additional elemental fluorine fluorination [61]. Elemental fluorine fluorination is carried out at temperature ranging from 0 to 30 oC, the process goes free from significant forming of destructive fluorination products with the target product yield reaching 97.5 mass.%. Along with polyfluorinated alkansulfofluorides we have studied the fluorination of alkanesulfofluorides. The yield of perfluoroalkanesulfofluorides is 90% for CH3SO2F and 82 % for C2F5SO2F. Obtaining method of perfluoroalkanesulfofluorides provides high yield for target product when using standard technological techniques and available raw materials, that gives us an opportunity to implement it on an industrial scale. Unlike ECF this method provides for period stability of the process and makes the energy costs significantly lower. 4. Production of Ozone-Friendly Chladones Hexafluoroethane obtaining methods are mainly based on power-consuming processes (pyrolysis of CHF3, CF2=CF2). Here we offer hexafluoroethane obtaining method out of tetrafluoroethylene by elemental fluorine fluorination in the medium of inert solvent [27,62]. Fluorination is carried out in the medium of perfluorocarbon liquid, containing 8-11 (mass.)% of stable perfluoromethyl-2-pentafluoroethyldifluoropropyl radical at 50-60 oC and fluorine : tetrafluoroethylene ratio = 1:1 - 1.1 mole. This approach is used at developing the obtaining method of octafluoropropane by fluorination of hexafluoropropene by elemental fluorine influencing hexafluoropropene at temperature ranging from -10 - to +30 oC in the medium of fluorocarbon liquid (perfluorolefines of composition of (CF3)2CFCR1=CR2CF3, where R1 = F, C2F5, CF(CF2)2; R2 = F, CF3, CF2CF2CF2CF3 or linear perfluorinated aliphatic compounds of CF3(CF2)CF common formula, where n = 5 - 10), containing 3-8 (mass.)%, stable 1,1,3,3-tetraperfluoromethyl-2-pentafluoroethyldifluoropropyl radical [62]. Due to high thermal effect the reaction of direct fluorination of hexafluoropropene (-658 KJ/mole) was carried out in the liquid perfluorocarbon medium inside a specially designed reactor with combined mixing device. It is stated, that during fluorination of hexafluoropropene a stable radical is formed, stable at 20 oC during one-year storage. The developed octafluoropropane (chladone -218, R-218) obtaining method is characterized by as much as five times higher relative productivity, than actual commercial method for gas-cycle fluorination at CoF3 [28, 63]. The selectivity by hexafluoropropene amounts to 96-99 %. The method has been implemented at pilot (experimental-industrial) plant equipped with 200 dm3 reactor. Low-waste, focused at large-tonnage production gas-cycle elemental fluorine fluorination technology of hexafluoropropene has been developed. R-218 is applied for refrigerating engineering and electronics and is an ozone-friendly product. n-Perfluoropentane [64] is obtained at high yield by elemental fluorine fluorination of n-pefluoropentene. Information on fluorination of trichloromethane using metals fluorides in the presence of phase-transfer catalyst in the solvent medium allowed to come across a trifluoromethane obtaining method [65]. It was determined, that fluorination of trichloromethane by alkali metals fluorides in the dimethyl diethyleneglycole ether at 120-150 oC results generally in total substitution of chlorine atoms for fluorine in a molecule. The content of trifluoromethane in gaseous products of fluorination reaches 91.7 % (vol.). We have conducted a comparative analysis for effectiveness of potassium, sodium, calcium, ammonium fluorides in reactions of haloidhydrocarbons fluorination reactions under conditions of phase-transfer catalysis. We have noticed a small effectiveness of potassium fluorides in the reaction with trichloroethylene. The suggested synthesis method and a reactor are successfully approved at an experimental-industrial scale. It was determined, that fluorination of trifluoromethane using fluorine, containing oxygen (0.05-0.2% mass.) over tetrafluoromethane at 100-400 oC in reactor filled with metallic nozzle made of copper, nickel and chrome results in formation of terafluoromethane (products' composition 99.8 % CF4, 0.2 % C2F6 ) [66]. It allowed increasing the conversion of raw materials and selectivity of the process and lowering the power inputs. Tetrafluoromethane obtaining method by fluorinating of different grades of coal containing activated carbon (from 5 to 25% mass.) at temperature ranging from 700 to 1200 oC was developed [67]. We shall mention, that fluorination is conducted either using pure fluorine or anode gas, formed during electrolysis of KF-2HF mixture and containing up to 10 (mass.) % of anhydrous hydrogen fluoride. It allows to exclude labour-intensive and power-consuming stage of fluorine purification. At the same time the presence of anhydrous hydrogen fluoride allows to depart from solvent of reaction mixture. The availability of anhydrous hydrogen fluoride results in decreasing of excessive heat coming from fluorinating reaction, improves heat exchange, increases the process selectivity due to lowering of formation of concurrent high-molecular perfluoroalkanes. The use of activated carbon additives decreases the process explosive risk, leads to combustion process faster reaching of the stable stage and, therefore to increasing of CF4 yield. The concentration of additive higher than 25(mass.)% makes the method economically irrational. Conclusion As can be seen from the data, listed in this review, the conception of synthesis of perfluorinated organic compounds by fluorination using elemental fluorine both in a pure form and in the presence of catalysts has found its experimental approval. We also should mark the importance of results of such approach decision not only and not as much as for obtaining methods of practically necessary perspective fluoroorganic compounds but for economical practicability. Thus, it is manageable to increase the yield of target perfluorinated compounds sharply, to improve their quality, to increase significantly the effectiveness of fluorine use and to simplify the process itself and obtaining technology of many substances. These results showed the urgency and timeliness of setting such a problem and a need for its boosted solving. The ideas, laid as a foundation of conception mentioned above, proved to be productive and their practical implementation for commercial production will stimulate a change in the production men's way of thinking regarding the question of using elemental fluorine for production. |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
1. Chemistry of Organic Fluorine Compound. II. Eds. M. Hydlicky, A.E. Pavlath/ Washington (D.C.) : Amer. Chem. Soc., 1995, 1296 c. (ACS Monography, N 187).
2. Enetani M., Esgmura T., V kn. Novoe v tekhnologii soedinenij ftora. Pod red. N. Isikava., per. pod red. A.V. Fokina.M. : Mir, 1984, s. 289-446
3. Organofluorine Chemistry. Principles and Commercial Application. Eds. Banks R.E., Smart B.E., Tatlow J.C., Plenum Publishing Corporation, 1994.
4. Rahimov A.I. Khimiya i tekhnologiya ftororganicheskih soedinenij. -M. : Khimiya, -1986, 272 s.
5. Moldavsky D.D., Furin G.G. J. Fluorine Chem., 1998, v. 87, p. 111-121.
6. Moldavsky D.D., Bispen T.A., Kaurova G.I., Furin G.G. J. Fluorine Chem., 1999, v. 94, p. 157-167.
7. Novye ftororuyushchie reagenty v organicheskom sinteze. Pod red. L.S.German, S.V.Zemsky, Novosibirsk : Nauka, 1987, 256 s.
8. Furin G.G., G.P. Gambaretto Direct Fluorination of Organic Compounds. CLEUP Cooperativa Libraria Editrice Universita di Padova, Italy, 1996, 212p. 9. Shimizu M., Hiyama T. Angew. Chem. Int. Ed. Engl., 2005, v. 44, N 2, p. 214-231.
10. Tavener S.J., Clark J.H. J. Fluorine Chem., 2003, v. 123, N 1, p. 31-36.
11. Drakesmith F.G. Top. Curr. Chem., 1997, v. 193, p. 197-242.
12. Shimizu M., Hiyama T. Angew. Chem. Int. Ed. Engl., 2004, v. 44 N 2, p. 214-231.
13. Suzuki Y., Watanabe K., Yanase K. (Asahi Glass Co., Ltd., Japan), PCT Int. Appl. WO 2004035518 (2004); Chem. Abstr., 2004, v. 140, 356951.
14. Tavener S.J., Clark J.H. J. Fluorine Chem., 2003, v. 123, N 1, p. 31-36.
15. Kirsch P., Hahn A. (Merck Pat. G.m.b.H., Germany) Ger. Offen DE 10258577 (2003); Chem. Abstr., 2004, v. 139, 133074.
16. Moldavski D.D., Kaurova G.T., Bispen T.A., Furin G.G. J. Fluorine Chem., 1993, v. 63, p. 193-201.
17. Shennard U., Sharts K. Organicheskaya khimiya ftora, M.: Mir, 1972, 480 s.
18. Lagow R.J., Margrave J.L. Direct Fluorination : A "New" Approach to Fluorine Chemistry. in : Progress in inorganic Chemistry/Ed. S.J.Lippard, -Interscience Publication, -John Wiley and Sons : New York, -1979, -v.26, -P.161-210.
19. Zaharov V.Yu.. Denisenkov A.K., Novikov M.D. // Zh.organ. khimii, 1994, t. 30, v. 12, s. 1844-1847.
20. Bispen T.A., Mihajlova T.V., Moldavskij D. D. i dr. // Zh. prikl. khimii, 1996, t. 69, v. 1, s. 112-119.
21. Advances in Fluorine Chemistry Eds. M.Stacty. J.C.Ta-tlow, A.G.Sharpe. London: Buttcrworths Sci. Publ., 1960-1961, v. 111, 570 p.
22. Bockemuller W. // Lieb. Ann., 1933, bd. 506, s. 20- 23.
23. Wilkinson J.A. // Chem. Rev., 1992, v. 92, p. 505-519.
24. Bispen T.A., Moldavskij D.D., Furin G.G. // Zh.prikl. khimii, 1998, t. 71, v. 8, s. 1334-1342; Chem. Abstr. 1999, v. 130, 211033.
25. Bispen T.A., Moldavskij D.D., Furin G.G. // Zh. prikl. khimii, 1998, t. 71, v 6. s. 977-981; Chem. Abstr., 1999, v. 130, 224586.
26. Kobayashi M., Ishii F., Tomioka H., Oka K. 12th European Symposium on Fluorine Chemistry. Aug. 29 – Sep. 2, 1998, Berlin, Germany. Anstracts, A36.
27. Tereshchenko G.F., Kuznetsov A.S., Il'in A.N., i dr. // Patent RU 2124493, Publ. 1999. Chem. Abstr., 2000, v. 133, 176946s.
28. Abramov O.B., Aleksandrova T.S., Araslanov G.G. i dr. Patent RU 2185363 (2002), Chem. Abstr., 2004, v. 139, 54551.
29.Watanabe K., Okazoe T., Tatematsu S. (Asahi Glass Co., Ltd., Japan) Eur. Pat. Appl. EP 967191 (1999); Chem. Abstr., 2000, v. 132, 51455.
30.Okada N., Kato M., Yoshinaga M. (Tokuyama Soda Co., Ltd., Japan) Jpn. Kokai Tokkyo Koho JP 09173773 (1997), CI, B 01 D 053-70; Chem. Abstr., 1998, v. 127, 98975.
31. Rodgers A.S. J. Phys. Chem., 1965, v. 69, N 1, p. 254-257.
32. Scherer K.V., Ono T., Jamanouchi J. Am. Chem. Soc., 1985, v. 107, N 3, p. 718-719.
33.Ono H., Nakajo T., Arai T., Oi T. (Showa Denko K.K., Japan), Jpn. Kokai Tokkyo Koho JP 09255598 (1997), CI, C 07 C 019-08; Chem. Abstr., 1998, v. 127, 331191.
34.Pashkevich D.S., Muhortov D.A., Alekseev Yu.T., Asovich V.S., Zh. prikl. khimii, 2002, t. 75, № 8, s. 1269-1274.
35.Seki E., Aoyama H.(Daikin Industries, Ltd., Japan) Jpn. Kokai Tokkyo Koho JP 11124352 (1999), CI, C 07 C 041-22; Chem. Abstr., 1999, v. 130, 337838.
36. Ono T., Yamanouchi K., Scherer K.V. J. Fluorine Chem., 1995, v. 73, p. 267-272.
37. Moldavskij D.D., Bispen T.A., Furin G.G., ZHuzhgov E.L. // Zh. prikl. khimii, 1996, t. 69, № 4, s, 636-644; Chem. Abstr., 1996, v. 69, p. 636-644.
38. Weinmaye V. // J. Org. Chem., 1963, v. 28, N 2, p. 492- 493.
39. Furin G.G., Dubovenko Z.D., Moldavskij D.D. // Zh. prikl.khimii, 1996, t. 69, № 1, s. 103-111.
40. Spravochnik khimika. t. 1 / Pod red. B.P.Nikol'skogo. L.: Khimiya, 1971, s. 925-930.
41. Moldavskij D.D., Shkul'tetskaya L.V., Furin G.G. 3-ya Mezhdunarodnaya konferentsiya «Khimiya, tekhnologiya i primenenie ftorsoedinenij» CTAF`2001.6-9 iyunya 2001, S. Peterburg, Russia. Tez. Dokl. R1-21, s. 156-157.
42. Oharu K., Takagi Y., Murotani E. (Asahi Glass Co., Ltd., Japan), Jpn. Kokai Tokkyo Koho JP 2004 143079 (Cl. C 07 C 51/60) (2004); Chem. Abstr., 2004, v. 140, 406548q.
43. Okazoe T., Watanabe K., Tatemasu S., Murofushi H. (Asahi Glass Co., Ltd., Japan), PCT Int. Appl. WO 2000 056694 (Cl. C 07 C 059-135), Chem. Abstr., 2000, v. 133, 266514.
44. Okazoe T., Watanabe K., Itoh M., Shirakawa D., Tatematsu S., Takagi H. (Asahi Glass Co., Ltd., Japan), PCT Int. Appl. WO 2002 018314 (Cl. C 07 C 049-167, C 07 C 051-58, C 07 C 059-135); Chem. Abstr., 2003, v. 136, 216751.
45. Fontana G., Navarrini W. (Solvay Solexis S.p.A.), WO 1440993 EU (2004).
46. Suzuki Y., Watanabe K., Yanase K., (Asahi Glass Co., Ltd., Japan) PCT Int. Appl. WO 2004 035518 (2004) CI, C 07 C 067-287; Chem. Abstr., 2004, v. 140, 356951.
47. Okazoe T., Murotani E., Watanabe K., Itoh M., Shirakawa D., Kawahara K., Kaneko I., Tatematsu S. J. Fluorine Chem., 2004, v. 125, p. 1695-1701.
48.Okamoto H., Okazoe T., Watanabe K., Takagi H. (Asahi Glass Co., Ltd., Japan) PCT Int. Appl. WO 2002 026679 (2002); Chem. Abstr., 2003, v. 136, 294533.
49. Okazoe T., Watanabe K., Itoh M., Shirakawa D., Kawahara K., Tatematsu S. J. Fluorine Chem., 2005, v. 126, p. 521-527.
50. MoldavskijD.D., FurinG.G. // Zh. obshch. khimii, 1996, t. 66, v. 12, s. 1995-2002; Chem. Abstr., v. 126, 225259.
51. Kornilov V.V., Kostevv R.A., Maksimov B.N., i dr. // Zh. prikl. khimii, 1995, t. 68, № 9, s. 1409-1418.
52. Stepej M., Tetpou D.K. Uspekhi khimii ftora. t. 1. M.: Khimiya, 1964, 576 s.
53 Zaharov V.Yu., Denisov A.K., Novikova M.D. // Zh. org. khimii, 1994, t. 30, № 12, s. 1844-1846.
54. Zaharov V.Yu., Denisov A.K., Novikova M.D. Tez. dokl. Mezhdunar. konf. «Khimiya, tekhnologiya i primenenie ftorsoderzhashchih soedinenij v promyshlennosti». SPb, Russia, 1994, s. 11.
55. Moldavskij D.D., Furin G.G., Shkul'tetskaya L.V., Eifman B.Ya. // Zh. prikl. khimii, 2000, t. 73, v. 6, s. 976-978; Chem. Abstr., 2004, v. 138, 204583.
56. Rozovskij A.Ya. Katalizator i reaktsionnaya sreda. M.: Nauka, 1988. 303 s.
57. Asovich B.C.. Kosteev R.A. // Zh. prikl. khimii, 1994, t. 67, № 8, s. 1320-1323.
58. Suhotin A.M., Zotikov B.C. Khimicheskoe soprotivlenie materialov, L.: Khimiya, 1975, s. 114.
59.Bispen T.A., Mihailova T.V., Moldavskij D.D. i dr. // Zh. prikl. khimii, 1996, t. 69, v. 1, s. 112-119.
60. Hansen J.C. Pat. U.S. 5474657 (1995).
61. Barabanov V.G., Borutskaya G.V., Bispen T.A., i dr. Patent RU 2237659 (2003).
62. Denisenkov V.F., Il'in A.N., Volkov V.N., i dr. // WO 93036358.04 Russia. Publ. 1996; Patent RU 2074162 Publ. 1997; Chem. Abstr., 1997, v. 127, 176171m.
63. Il'in A.N., Ivanova L.M. // Mezhdunarodnaya nauchno-tekhnicheskaya konferentsiya «Perspektivy himicheskoj tekhnologii i materialov. Perm' [1997] : Tez. Dokl. – Perm', 1997, c. 203; RZhKhim. 1998. 12N 130.
64. Watanabe K., Okazoe T., Tatematsu S. (Asahi Glass Co., Ltd., Japan) , Eur. Pat.Appl. EP 967191 (1999); Chem. Abstr., 2000, v. 132, 51455.
2. Enetani M., Esgmura T., V kn. Novoe v tekhnologii soedinenij ftora. Pod red. N. Isikava., per. pod red. A.V. Fokina.M. : Mir, 1984, s. 289-446
3. Organofluorine Chemistry. Principles and Commercial Application. Eds. Banks R.E., Smart B.E., Tatlow J.C., Plenum Publishing Corporation, 1994.
4. Rahimov A.I. Khimiya i tekhnologiya ftororganicheskih soedinenij. -M. : Khimiya, -1986, 272 s.
5. Moldavsky D.D., Furin G.G. J. Fluorine Chem., 1998, v. 87, p. 111-121.
6. Moldavsky D.D., Bispen T.A., Kaurova G.I., Furin G.G. J. Fluorine Chem., 1999, v. 94, p. 157-167.
7. Novye ftororuyushchie reagenty v organicheskom sinteze. Pod red. L.S.German, S.V.Zemsky, Novosibirsk : Nauka, 1987, 256 s.
8. Furin G.G., G.P. Gambaretto Direct Fluorination of Organic Compounds. CLEUP Cooperativa Libraria Editrice Universita di Padova, Italy, 1996, 212p. 9. Shimizu M., Hiyama T. Angew. Chem. Int. Ed. Engl., 2005, v. 44, N 2, p. 214-231.
10. Tavener S.J., Clark J.H. J. Fluorine Chem., 2003, v. 123, N 1, p. 31-36.
11. Drakesmith F.G. Top. Curr. Chem., 1997, v. 193, p. 197-242.
12. Shimizu M., Hiyama T. Angew. Chem. Int. Ed. Engl., 2004, v. 44 N 2, p. 214-231.
13. Suzuki Y., Watanabe K., Yanase K. (Asahi Glass Co., Ltd., Japan), PCT Int. Appl. WO 2004035518 (2004); Chem. Abstr., 2004, v. 140, 356951.
14. Tavener S.J., Clark J.H. J. Fluorine Chem., 2003, v. 123, N 1, p. 31-36.
15. Kirsch P., Hahn A. (Merck Pat. G.m.b.H., Germany) Ger. Offen DE 10258577 (2003); Chem. Abstr., 2004, v. 139, 133074.
16. Moldavski D.D., Kaurova G.T., Bispen T.A., Furin G.G. J. Fluorine Chem., 1993, v. 63, p. 193-201.
17. Shennard U., Sharts K. Organicheskaya khimiya ftora, M.: Mir, 1972, 480 s.
18. Lagow R.J., Margrave J.L. Direct Fluorination : A "New" Approach to Fluorine Chemistry. in : Progress in inorganic Chemistry/Ed. S.J.Lippard, -Interscience Publication, -John Wiley and Sons : New York, -1979, -v.26, -P.161-210.
19. Zaharov V.Yu.. Denisenkov A.K., Novikov M.D. // Zh.organ. khimii, 1994, t. 30, v. 12, s. 1844-1847.
20. Bispen T.A., Mihajlova T.V., Moldavskij D. D. i dr. // Zh. prikl. khimii, 1996, t. 69, v. 1, s. 112-119.
21. Advances in Fluorine Chemistry Eds. M.Stacty. J.C.Ta-tlow, A.G.Sharpe. London: Buttcrworths Sci. Publ., 1960-1961, v. 111, 570 p.
22. Bockemuller W. // Lieb. Ann., 1933, bd. 506, s. 20- 23.
23. Wilkinson J.A. // Chem. Rev., 1992, v. 92, p. 505-519.
24. Bispen T.A., Moldavskij D.D., Furin G.G. // Zh.prikl. khimii, 1998, t. 71, v. 8, s. 1334-1342; Chem. Abstr. 1999, v. 130, 211033.
25. Bispen T.A., Moldavskij D.D., Furin G.G. // Zh. prikl. khimii, 1998, t. 71, v 6. s. 977-981; Chem. Abstr., 1999, v. 130, 224586.
26. Kobayashi M., Ishii F., Tomioka H., Oka K. 12th European Symposium on Fluorine Chemistry. Aug. 29 – Sep. 2, 1998, Berlin, Germany. Anstracts, A36.
27. Tereshchenko G.F., Kuznetsov A.S., Il'in A.N., i dr. // Patent RU 2124493, Publ. 1999. Chem. Abstr., 2000, v. 133, 176946s.
28. Abramov O.B., Aleksandrova T.S., Araslanov G.G. i dr. Patent RU 2185363 (2002), Chem. Abstr., 2004, v. 139, 54551.
29.Watanabe K., Okazoe T., Tatematsu S. (Asahi Glass Co., Ltd., Japan) Eur. Pat. Appl. EP 967191 (1999); Chem. Abstr., 2000, v. 132, 51455.
30.Okada N., Kato M., Yoshinaga M. (Tokuyama Soda Co., Ltd., Japan) Jpn. Kokai Tokkyo Koho JP 09173773 (1997), CI, B 01 D 053-70; Chem. Abstr., 1998, v. 127, 98975.
31. Rodgers A.S. J. Phys. Chem., 1965, v. 69, N 1, p. 254-257.
32. Scherer K.V., Ono T., Jamanouchi J. Am. Chem. Soc., 1985, v. 107, N 3, p. 718-719.
33.Ono H., Nakajo T., Arai T., Oi T. (Showa Denko K.K., Japan), Jpn. Kokai Tokkyo Koho JP 09255598 (1997), CI, C 07 C 019-08; Chem. Abstr., 1998, v. 127, 331191.
34.Pashkevich D.S., Muhortov D.A., Alekseev Yu.T., Asovich V.S., Zh. prikl. khimii, 2002, t. 75, № 8, s. 1269-1274.
35.Seki E., Aoyama H.(Daikin Industries, Ltd., Japan) Jpn. Kokai Tokkyo Koho JP 11124352 (1999), CI, C 07 C 041-22; Chem. Abstr., 1999, v. 130, 337838.
36. Ono T., Yamanouchi K., Scherer K.V. J. Fluorine Chem., 1995, v. 73, p. 267-272.
37. Moldavskij D.D., Bispen T.A., Furin G.G., ZHuzhgov E.L. // Zh. prikl. khimii, 1996, t. 69, № 4, s, 636-644; Chem. Abstr., 1996, v. 69, p. 636-644.
38. Weinmaye V. // J. Org. Chem., 1963, v. 28, N 2, p. 492- 493.
39. Furin G.G., Dubovenko Z.D., Moldavskij D.D. // Zh. prikl.khimii, 1996, t. 69, № 1, s. 103-111.
40. Spravochnik khimika. t. 1 / Pod red. B.P.Nikol'skogo. L.: Khimiya, 1971, s. 925-930.
41. Moldavskij D.D., Shkul'tetskaya L.V., Furin G.G. 3-ya Mezhdunarodnaya konferentsiya «Khimiya, tekhnologiya i primenenie ftorsoedinenij» CTAF`2001.6-9 iyunya 2001, S. Peterburg, Russia. Tez. Dokl. R1-21, s. 156-157.
42. Oharu K., Takagi Y., Murotani E. (Asahi Glass Co., Ltd., Japan), Jpn. Kokai Tokkyo Koho JP 2004 143079 (Cl. C 07 C 51/60) (2004); Chem. Abstr., 2004, v. 140, 406548q.
43. Okazoe T., Watanabe K., Tatemasu S., Murofushi H. (Asahi Glass Co., Ltd., Japan), PCT Int. Appl. WO 2000 056694 (Cl. C 07 C 059-135), Chem. Abstr., 2000, v. 133, 266514.
44. Okazoe T., Watanabe K., Itoh M., Shirakawa D., Tatematsu S., Takagi H. (Asahi Glass Co., Ltd., Japan), PCT Int. Appl. WO 2002 018314 (Cl. C 07 C 049-167, C 07 C 051-58, C 07 C 059-135); Chem. Abstr., 2003, v. 136, 216751.
45. Fontana G., Navarrini W. (Solvay Solexis S.p.A.), WO 1440993 EU (2004).
46. Suzuki Y., Watanabe K., Yanase K., (Asahi Glass Co., Ltd., Japan) PCT Int. Appl. WO 2004 035518 (2004) CI, C 07 C 067-287; Chem. Abstr., 2004, v. 140, 356951.
47. Okazoe T., Murotani E., Watanabe K., Itoh M., Shirakawa D., Kawahara K., Kaneko I., Tatematsu S. J. Fluorine Chem., 2004, v. 125, p. 1695-1701.
48.Okamoto H., Okazoe T., Watanabe K., Takagi H. (Asahi Glass Co., Ltd., Japan) PCT Int. Appl. WO 2002 026679 (2002); Chem. Abstr., 2003, v. 136, 294533.
49. Okazoe T., Watanabe K., Itoh M., Shirakawa D., Kawahara K., Tatematsu S. J. Fluorine Chem., 2005, v. 126, p. 521-527.
50. MoldavskijD.D., FurinG.G. // Zh. obshch. khimii, 1996, t. 66, v. 12, s. 1995-2002; Chem. Abstr., v. 126, 225259.
51. Kornilov V.V., Kostevv R.A., Maksimov B.N., i dr. // Zh. prikl. khimii, 1995, t. 68, № 9, s. 1409-1418.
52. Stepej M., Tetpou D.K. Uspekhi khimii ftora. t. 1. M.: Khimiya, 1964, 576 s.
53 Zaharov V.Yu., Denisov A.K., Novikova M.D. // Zh. org. khimii, 1994, t. 30, № 12, s. 1844-1846.
54. Zaharov V.Yu., Denisov A.K., Novikova M.D. Tez. dokl. Mezhdunar. konf. «Khimiya, tekhnologiya i primenenie ftorsoderzhashchih soedinenij v promyshlennosti». SPb, Russia, 1994, s. 11.
55. Moldavskij D.D., Furin G.G., Shkul'tetskaya L.V., Eifman B.Ya. // Zh. prikl. khimii, 2000, t. 73, v. 6, s. 976-978; Chem. Abstr., 2004, v. 138, 204583.
56. Rozovskij A.Ya. Katalizator i reaktsionnaya sreda. M.: Nauka, 1988. 303 s.
57. Asovich B.C.. Kosteev R.A. // Zh. prikl. khimii, 1994, t. 67, № 8, s. 1320-1323.
58. Suhotin A.M., Zotikov B.C. Khimicheskoe soprotivlenie materialov, L.: Khimiya, 1975, s. 114.
59.Bispen T.A., Mihailova T.V., Moldavskij D.D. i dr. // Zh. prikl. khimii, 1996, t. 69, v. 1, s. 112-119.
60. Hansen J.C. Pat. U.S. 5474657 (1995).
61. Barabanov V.G., Borutskaya G.V., Bispen T.A., i dr. Patent RU 2237659 (2003).
62. Denisenkov V.F., Il'in A.N., Volkov V.N., i dr. // WO 93036358.04 Russia. Publ. 1996; Patent RU 2074162 Publ. 1997; Chem. Abstr., 1997, v. 127, 176171m.
63. Il'in A.N., Ivanova L.M. // Mezhdunarodnaya nauchno-tekhnicheskaya konferentsiya «Perspektivy himicheskoj tekhnologii i materialov. Perm' [1997] : Tez. Dokl. – Perm', 1997, c. 203; RZhKhim. 1998. 12N 130.
64. Watanabe K., Okazoe T., Tatematsu S. (Asahi Glass Co., Ltd., Japan) , Eur. Pat.Appl. EP 967191 (1999); Chem. Abstr., 2000, v. 132, 51455.
65. Il'in A.N., Ivanova L.M. VI Vsesoyuznaya konferentsiya po khimii ftororganicheskih soedinenij. 26-28 iyunya 1990. Novosibirsk. Tezisy dokladov, s. 194.
66. Uklonskij I.P., Denisenkov V.F., Il'in A.N., i dr. // Patent RU. 2181352, Publ. 2001. B.I. № 11. (2002).
67. Uklonskij I.P., Denisenkov V.F., Il'in A.N., i dr. // Patent RU 2181351, Publ. 2001; B.I. № 11. (2002).
Fluorine Notes, 2007, 52, 3-4
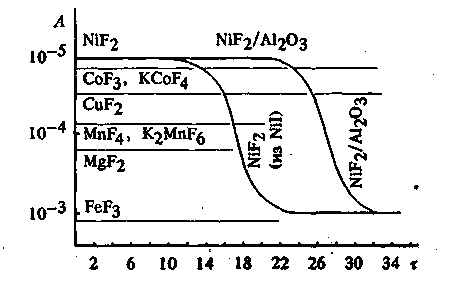
 - process period (h) )
- process period (h) )