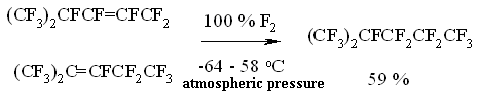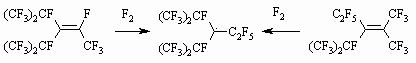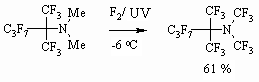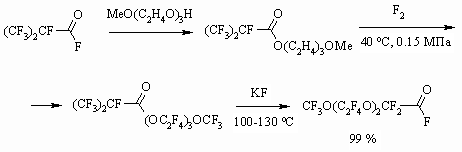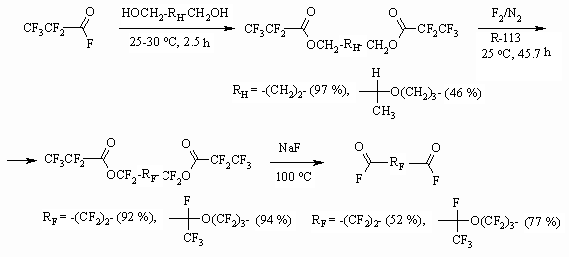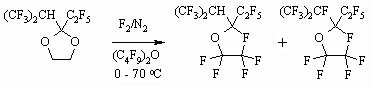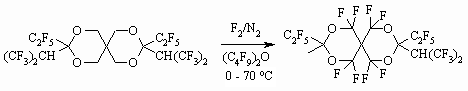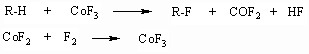Fluorine Notes, 2007, 51, 1-2
ADDITIONAL FLUORINATION OF PARTLY FLUORINATED ORGANIC COMPOUNDS BY ELEMENTAL FLUORINE AS AN OBTAINING METHOD FOR PRACTICAL PURPOSE FLUORINE MATERIALSD.D. Moldavsky, G.G. Furin* RSC "Applied Chemistry" 14, Dovrolubov av., Saint-Petersburg, 197198 * Novosibirsk Institute of Organic Chemistry , Russian Academy of Science 630090, Novosibirsk, Ac. Lavrentiev av. 9 (continuation) 1. Organic Compounds Elemental Fluorine Direct Fluorination At hydrocarbons' elemental fluorine fluorination the yield of perfluorinated analogues doesn't usually exceed few percents, low-molecular fluorine containing paraffin's are main products, what is explained by high energy of forming C-F (448-507 KJ/mole) bond which exceeds the C-C (347 KJ/mole) bond energy and leading to fragmentation of hydrocarbonic skeleton [16-18]. High heat of reaction combined with relatively low dissociation energy of fluorine [D(F-F) 155 KJ/mole] results in quick branching of chain process, and if the heat doesn't leak immediately, then the inflammation occurs. At the same time using of partly fluorinated compounds as starting materials results in obtaining perfluorinated analogues with rather high yield [19, 20]. It's not surprising, because as the rate of substitution of hydrogen atoms by fluorine increases the molecule deactivates itself more and more. Using special techniques (fluorination applying low-temperature gradient, diluting of fluorine by 25-100 times, aerosol fluorination) allows obtaining of perfluorinated products of satisfactory yield, though their productivity is low, they are complicated to be implemented for apparatus, and do not go beyond laboratory frames. Along with that in the work [21] it is displayed, that fluorine containing hydrocarbons of high yield can be finally fluorinated by elemental fluorine to perfluorinated ones. At that fluorination doesn't require special mild conditions: the temperature floats within the range starting from -30 (Fluorination of lowest olefins) ending 440oC (Fluorination of mono- and dihydroperfluorinated compounds which number of carbon atoms is in between 12-15). The fluorination process itself could have been a reason, and namely fluorination on the gas-liquid separating surface. Liquid-phase fluorination is being studied rather widely [21]. Here information on fluorine solubility in organic solvent gets its importance. Fluorination occurs at fluorine bubbling through hydrocarbon soluted in inert solvent. Many authors consider, that during these processes the reaction goes in gas phase (in bubbles). The author of the work [22] used such construction of the apparatus, that provided the prolonged contact between gas (elemental fluorine) and liquid, modeling the process of fluorination on the gas-liquid separation surface. At that he managed to carry out the direct fluorination of hydrocarbons in the temperature range of 0-20oC. It was stated [23,24], that as fluorine substitutes hydrogen the fluorinating rate decreases; at that not only the heat of fluorination is decreasing, but the emission of heat as well, which makes it possible in principle to use typical technological equipment for carrying out the fluorinating processes. Calculations [24] prove us, that thermal effects of the reaction increase during transfer from perfluorinated olefines to partly fluorinated compounds of other classes with the same number of carbon atoms; fluorination specific heat decreases as fluorinated chain becomes longer (Table 1). [24]. Table 1. Calculated Heats of Fluorination of Some Fluorine Containing Compounds
1.1. Direct Fluorination of Fluoroolefines Now we will proceed to consider some approaches regarding improving the fluorinating process itself, realization of fluorination using high concentrated fluorine (approximately 100%), approaches regarding the purification of target products from partly fluorinated compounds and regarding production of high purity perfluorinated compounds. Fluorinations of perfluorinated olefines and partly fluorinated paraffins, ethers and trialkylamines using elemental fluorine under conditions of contact of gaseous 100% fluorine with surface of liquid is described in the work [25]. In Table 2 the information regarding some olefines fluorination in gas phase is presented. Table 2. Fluorination of Perfluorinated Olefines During Gas Phase (Contact Period 40 min) [6]
a - compound [ (CF3)2CF]2CFC2F5, According to Table 2 we can conclude, that as hydrocarbonic chain becomes longer the fluorinating conditions become "more and more severe". Thus, C1-C4 perfluorinated olefins are being fluorinated at -40 - -10oC, while C8-C12 olefins - at 120-160 oC, the fluorination period rises from 0,5 to 45 hours. It can be associated with values of oscillating and rotational energy of molecule as a whole, which depends on carbon chain length. Fluorinating conditions become severer as molecules become saturated with fluorine. Fluorination of perfluorinated olefins with long carbon chain goes under severer conditions compare to hexafluoropropylene, which can be explained by the influence of spatial factors. As chain becomes longer, the spatial screening of multiple bond occurs, because the molecules of perfluorolefines are anfractuous and not linear. An additional energy is required to turn the molecule into the linear one, that provokes the approaching of multiple bond fluorine. Another reason for that is in spatial screening of initial radical center by perfluoroalkyl radicals. As the size of chain increases the radical center screening grows, the energy spent for oscillations and rotations of atoms in RF group, which makes the fluorinating conditions severer. In table 2 you can find the main results of fluorination of some perfluorinated olefins in gas phase. You can see by the table data, that as concentration of initial fluoroolefine decreases the fluorinating rate drops and to finish it we need to increase the temperature; at that, the yield of destructive fluorination products - perfluorinated paraffins of shortened carbon chain increases. In spite of that, we observe the high yield of target pefluorinated paraffins. Although using hexafluoropropene as solvent decreases the destructive processes compare to process in gas phase, it doesn't suppress them on a whole, which can be connected with high concentration of fluorine atoms, caused by olefine excess. In Table 3 you can see the results of hexafluoropropene fluorination in gas-phase and different solvents. As you can see by the Table 3 data the process carrying out in gas-phase makes destructive processes rather notable not providing the total conversion. Table 3. Conditions and Composition of Hexafluoropropene Elemental Fluorine Fluorination Products [6]
Nevertheless, the process is rather simple for technological application and doesn't require special conditions and equipment and it can be a base for commercial obtaining technology for perfluorinated paraffins. We can use the production technology of perfluoropentene and perfluoro -2-methylpentane by elemental fluorine fluorination of perfluoropenten-2-ene and perfluoro-4-methylpent-2-ene, which was implemented at an experimental scale at Experimental Plant of RSC of Applied Chemistry, as an example. We should note, that authors themselves had carried out the successful fluorination of hexafluoropropylene dimers using 100% fluorine in liquid phase [26].
The fluorination of tetrafluoroethylene [27], hexafluoropropene [28], 2-fluoropentene [29] had been carried out the same way. It's better to carry out the process in inert to fluorine solvents, which can be the products of fluorination themselves. In the work [30] it is suggested to use elemental fluorine for detoxication of extremely toxic octafluoroisobutylene, which is generated during pyrolysis of elastomers based on tetrafluoroethylene, instead of perfluorotripentylamine applied for these purposes. The ease of fluorine adding to multiple C=C bonds is to a great extent determined by steric factor influence. Thus, fluorine adding by weakly screened multiple bond of tetrafluoroethylene is going easy, though perfluoro-2-butene here requires higher temperature of reaction. [31], and strongly screened multiple bond of perfluoro(1-isopropyl-1-ethyl-2,2-dimethyl)- and (1,1-diisopropyl-2methyl)-ethylenes is on the whole inert to fluorine. Fluorination of these olefins at 300 K stops at the stage of forming of stable (perfluorodiisopropyl-ethyl)methyl radical, which can be distilled without decomposition[32].
1.2. Direct Fluorination of Partly Fluorinated Paraffins, Dialkyl Ethers and Trialkyl Amines Final fluorination of CHF3 and CF3CH2F by gaseous fluorine requires high temperature (400 and 370 oC respectively) and pressure close to 1.5 MPa (Table 4) [6,33]. Different fluoroethanes are also finally fluorinated using elemental fluorine [34]. Table 4. Fluorination of Polyfluorinated Paraffins using 100 % [6]
Dialkyl ethers are more resistant to fluorine, than paraffins, olefins and trialkylamines (Table 5), which probably can be connected to the oxidation level of molecules ( dimethyl ether is fluorinated up to CF3OCF3 of 33% yield, while ethylene - up to hexafluoroethane of 7.5% yield) [6]. Table 5. Fluorination of Partly Fluorinated Ordinary Ethers [6]
However, it should be noted that during final fluorination of partly fluorinated ethers the process is going not selectively enough. Thus, the fluorination of HCF2CF2OMe using elemental fluorine both in the solution of anhydrous hydrogen fluoride and in the solution of chlorotrifluoroethylene oligomer oil at room temperature produces mixture of ethers, containing different number of fluorine atoms [35]. Trialkylamines, containing even one perfluoroalkenyl radical are less resistant to fluorine influence, that is due to highest thermal effect of the reaction: in most favorable case 6 atoms of hydrogen undergo substitution (Table 6). Table 6. Polyfluoroalkylamines Fluorination Using Elemental Fluorine [6]
a Fluorination reaction in CF3N(C2F5)2
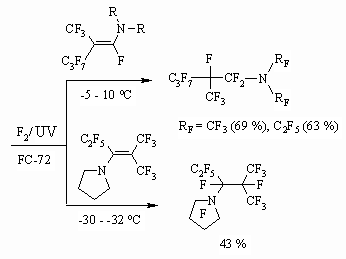
(10 %) solution To conduct a successful fluorination we need mild conditions: low temperature, solvent, effective stirring and long period for the process. At fluorinating of amines and ethers, containing multiple bonds in alkyl chain, at first, multiple bond is being fluorinated and only then hydrogen atoms are being substituted. While transferring from paraffins to dialkyl ethers the selectivity of fluorination is growing: while fluorinating dihydroperfluorinated paraffins the content of monohydroderivatives is not high after feeding the 1 mole of fluorine and their content in products of reaction is about 10%, whereas after feeding the half quantity of fluorine into α,α'-dimethyl ether the content of products of partial substitution is 68%. It is stated, that at fluorination of fluorine containing olefines, paraffins and ethers the yield of perfluorinated species is 55-99 %. At fluorination of fluorine containing tertiary amines with two alkyl groups the yield of tertiary perfluorinated amines amounts to 29-35%. As molecule is being saturated with fluorine and as fluorine containing chain is growing the fluorinating conditions are becoming severer. At transfer from polyfluorinated olefines and paraffins to fluorine containing ethers the selectivity of fluorination is growing. High heat of fluorination of N,N-dialkylamino N-perfluoroalkylenes provokes high compare to other fluorine containing compounds dissociation of fluorine molecules, that results in higher molecule's fragmentation. The yield of perfluoro-N,N-dimethylhexanes and N,N-dimethylnonanes are 47 and 55% respectively, while at fluorination of hexafluoropropene the yield of octafluoropropane is practically quantitative [25]. High emission of heat at obtaining perfluorinated compounds is a main problem when working out their obtaining technology of elemental fluorine direct fluorination. Nevertheless even N,N-dimethyl-N-perfluoroalkylenes are fluorinated using elemental fluorine forming corresponding tertiary perfluorinated amines which yield is as minimum as twice exceeding the yield obtained at electrochemical fluorination. Thus, N,N-dialkyl-N-perfluoroalkylenes are subject to liquid-phase fluorination using elemental fluorine at photochemical irradiation forming fully fluorinated trialkylamines [36].
This approach is being implemented as well for partly fluorinated trialkylamines. For example, the formation of [36] perfluoro(N,N-dimethyl-1,1-dimethylbutyl)amine is stated at direct elemental fluorine fluorination of 2-dimethylaminoperfluoro-2-methylpentane [36].
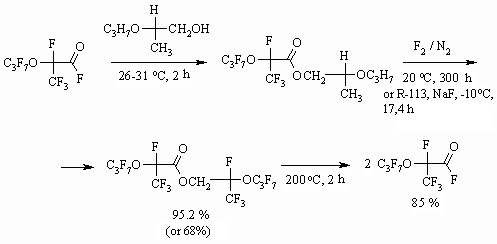
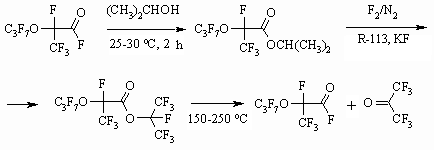
Synthesis of partly fluorinated derivatives doesn't produce any problems and is based on available and produced by the industry perfluoroolefines [37-39]. The obtained results show, that the developed methodology of fluorinating organic compounds on the gas-liquid contact surface using 100% elemental fluorine allow obtaining perfluorinated compounds with satisfactory yields, applying usual technological fluorinating techniques. The application of inert solvents decreases destructive processes largely, that is connected to more than tenfold increasing of the reaction mass heat conduction [40], absence of local overheating at the point of components' contact. When transferring from solvents of longer carbon chain to solvents of shorter carbon chain we observe an additional suppression of destructive processes, that may be connected to higher fluorine solubility in solvents of shorter carbon chain [5,41] and additional improving of heat-exchange. 1.3. Direct Fluorination of Carbonyl Containing Compounds One of the new ways of synthesis for perfluorinated carbonic acids is based on the following approach. At first we carry out the etherification of available and reasonably priced perfluorinated acid using aliphatic alcohol, afterwards the ester is subjected to liquid-phase fluorination by elemental fluorine forming fully fluorinated ester of the corresponding acid. It is decomposed by alkali metal fluoride at 100-130 oC forming two acids, which are being separated using distillation. The yield of target products by this method is much higher and it is an alternative for electrochemical fluorination of carboxylic acids [42-46].
Here it is very important to choose the appropriate perfluorinated carboxylic acid, which can be easily isolated out of mixture of acids. For example, acids' fluoroanhydrides, obtained at dimerization and trimerization of hexafluoropropylene oxide are widely used [43, 46, 47]. The presented method can be carried out at an industrial scale.
In case of using isopropyl alcohol for this reaction the hexafluoroacetone is formed as a second product [48]. Dissociation of ester bonds is carried out in the temperature range of 150-250 oC in the liquid phase without solvent using or during the gaseous phase in a tube, filled with glass, alkali or alkali-land metal for example KF, NaF or activated carbon [44].
Glycols were introduced into the reaction as well, that allows us to obtain perfluorinated dicarboxylic acids and their derivatives [49].
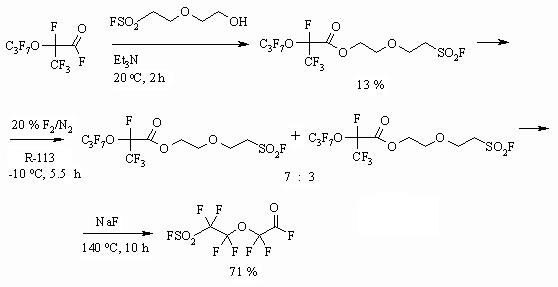
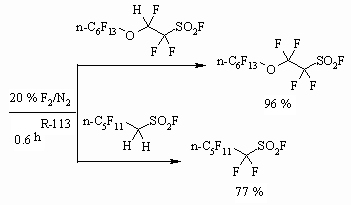
In case of alcohols, possessing SO2F ending group the fluorination using elemental fluorine is accompanied by substitution of this group by fluorine atom and depend on the length of carbon chain [47].
At the same time during influence of elemental fluorine on polyfluorosulphoacids fluoroanhydrides prove to be resistant and exchange of SO2F group for fluorine atom doesn't take place but all the hydrogen atoms are being substituted [47].
1.4 Heterocyclic Compounds Fluorination In case of partly fluorinated heterocyclic compounds the influence of fluorine diluted by nitrogen allow obtaining of fully fluorinated compounds. Let's draw just a few examples. Thus, fluorination of dioxalane using elemental fluorine, diluted by nitrogen (1:8), is going smoothly forming mixture of compounds at consecutive rising of temperature from 0 to 70oC in the solution of perfluorodibutyl ester. The hydrogen atom located by the tertiary carbon atom is the most difficult one for the fluorine atom to displace. Severe conditions and excess of fluorine are required to prevent such objects [50].
The fluorination of 1,3-dioxane derivative is carried out in the perfluorodibutyl ester as well and perfluorinated derivative of 2,4,8,10-tetraoxaspiro[5.5]undecane is produced [50].
1.5. Catalytic Method For Carrying Out The Purification of Mixtures Of Partly Fluorinated Compounds Cobalt trifluoride is a traditional fluorinating agent for obtaining (incl. commercial production) perfluorinated organic compounds, especially, parafines and ethers out of hydrocarbon and unsaturated analogues [51, 52]. In the reaction process cobalt trifluoride is being restored to difluoride:
Fluorinating using cobalt trifluoride is characterized by comparatively low target products' yield (30-40%), by limited productivity, caused by insignificant concentration of "activated" fluorine (16.30 wt. %; actual using is 1.2-3 times less) and also by noticeable residue content of hydrogen containing and unsaturated compounds (up to 1 wt. %) when the requirements of medicine, radioelectronics and other branches of industry are 1 .10-3 - 5.10-4 wt %. These particularities make us to look for other perfluorinated compounds' obtaining methods. Catalytic fluorination of fluorine containing organic compounds using elemental fluorine is one of them. Higher metals fluorides of variable oxidation including nickel, copper and cobalt fluorides [6,53-54] are used as catalysts. In the work [55] a comparative fluorination of monohydroperfluorotripropylamine C3F6HN(C3F7)2 (I) and monohydroperfluorotributylamine C4F8HN(C4F9)2 (II) using cobalt trifluoride and elemental fluorine in the presence of cobalt trifluoride had been studied. Compounds (I), (II) are main technological admixtures at electrochemical obtaining of perfluorotripropylamine (C3F7)3N and perfluorotributylamine (C4F9)3N respectively. Concentration of these admixtures in "crude material" reaches 30 wt %. Fluorination was carried out in the reactor, filled with CoF3, at that in first case the mixture of fluorinated compound's vapours and fluorine was put into the reactor, and in the second one the nitrogen was fed into the reactor instead of fluorine at the same rate. In Table 7 you can see the results of catalytic fluorination of some hydrogen containing and unsaturated compounds, which can be found in obtained by Electrochemical Fluorination (ECF) method "crude material" as admixtures. Table 7. Fluorination of Some Fluorine Hydrogen Containing and Unsaturated Compounds Using Elemental Fluorine at CoF3 [5]
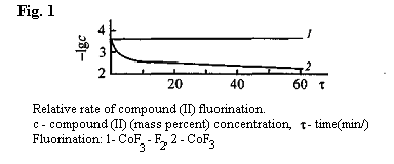
A*- residual content of unsaturated and hydrogen containing compounds It is illustrated by the results of fluorination of CoF3 and compound (II) at different temperature (Table 8 and Fig. 1). Table 8. Final Catalytic Fluorination of Polufluorinated Compounds Solutions [24]
Table 9. Fluorination of CoF3 Hydrogen Containing Admixtures of Perfluorotributylamine (contact period is 5 min., start concentration (C4HF8)3N is 17.5 mass.%) [55]
In Table 9 you can find information on CoF3 fluorinating process temperature impacts using an examples of hydrogen containing admixtures of perfluorotributylamine, obtained by ECF method. It is obvious, that temperature of 400oC is most effective [56].
Curve 2, showing the fluorinating rate of compound (II) CoF3 is typical for reactions, which starting stage goes in fact on the surface of one reagent (in the present case - CoF3) in the external kinetic area. As CoF3 is being consumed in the surface layer the reaction is transferring to the diffusion area, with what the seen low drop of the reaction rate is connected [56]. It should be noted, that the starting part of the curve 2 is rather difficult to be analyzed and the majority of researches studying the kinetics of fluorination of CoF3 hydrocarbons are usually limited by diffusion area, in which the rate lowering is being ignored and taken as constant one [56, 57]. At elemental fluorine fluorination in the presence of CoF3 (right line 1) the fluorination is going on a standard basis in the external kinetic area due to constant concentration of CoF3 in the surface layer, which is caused by on-time or even anticipatory fluorine inflow of the fluorine:
The results obtained make the difference in CoF3 and elemental fluorine with CoF3 as catalyst fluorinating processes obvious. In first case the fluorinating rate is low and decreases as time goes by, the process is limited by concentration of "activated" fluorine, that hampers its carrying out using uninterrupted pattern; the residual content of hydrogen containing or unsaturated compounds in final products is quite high (1.10-3 wt %), and that requires their additional purification. In second case, the rate of fluorination is high and constant the process is not limited by the time period in fact, which allows us to work using the uninterrupted pattern; the residual content of hydrogen containing compounds is less than 1 . 10-3 wt % (Catalytic fluorination process has been carried out at Pilot Plant of RSC of "Applied Chemistry").
to be continued |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
Fluorine Notes, 2007, 51, 1-2