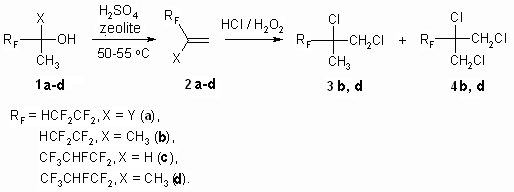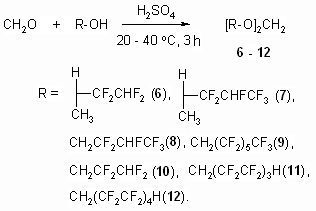Fluorine Notes, 2007, 50, 1-2
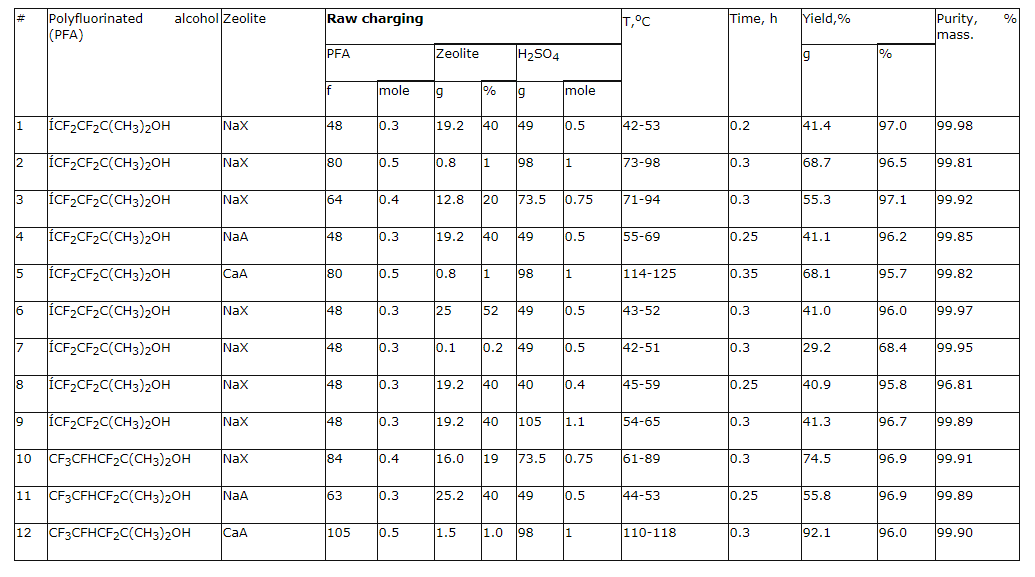
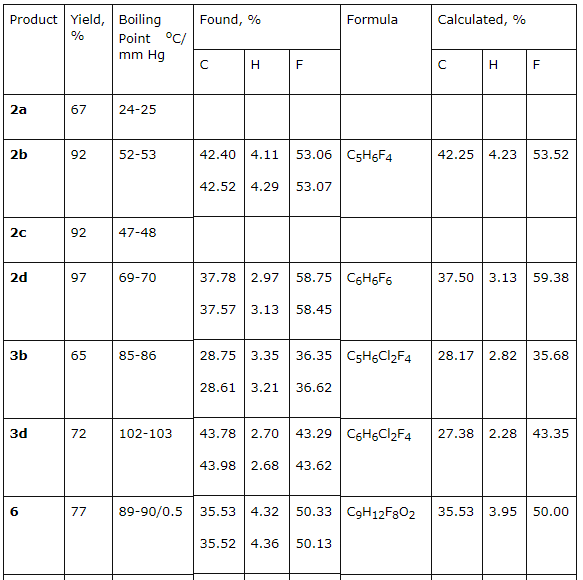
PARTLY FLUORINATED ALCOHOLS AS SEMI-PRODUCTS FOR SYNTHESIS OF FLUOROORGANIC COMPOUNDSG.G. Furin, A.A. Ilyin*, U.L. Bakhmutov*, A.N. Ilyin*, L.M. Ivanova* Novosibirsk Institute of Organic Chemistry , Russian Academy of Science 630090, Novosibirsk, Ac. Lavrentiev av. 9, E-mail : furin@nioch.nsc.ru * JSC "Halogen", Lasvinskaya St. 98, 614113, Perm, Russia E-mail: lexaaa@yandex.ru Here we display the opportunities of synthesis of perfluoroalkyl substituted ethylene and propylene by dehydration of secondary and tertiary fluorocontaining alcohols by influence of concentrated sulphuric acid in the presence of zeolites of types "NaA", "NaX" and acetales by formaldehyde influence in concentrated sulphuric acid. Products of chlorine addition to multiply bond are obtained while chlorinating the polyfluoroalkyl derivatives of ethylene and propylene at 25C. Fluorine containing materials with their unique complex of characteristics typical only for this particular class have their special status among organic compounds due to which find wide practical application [1]. In recent years the researchers' and manufacturers' interest is turned for fluorine alcohols [2-4], which proved themselves as universal products, used as solvents, anesthetic agents, plasticizing agents, surface active materials, dispersing agents, but as well as new starting materials necessary to obtain new construction materials, inert heat-transfers, lubricants, high-performance fire extinguishing compositions etc [5,6]. An important part is given to modifying (using them) of different types of monomers that to a great extent allows changing complex of their physical and chemical properties by doing so widening options of application of such polymeric materials [6]. The researching of option of using fluorine alcohols as effective O-nucleophilers showed their practical sense as independent source of raw materials for fluoroorganic synthesis. Throughout learning of these options of fluorine alcohols we here determine our task as using them to create high-performance production of perfluoroalkyl ethylene and propylene derivatives and fluorine containing acetales as well, which are promising starting compounds for synthesis of different fluorine containing materials. At that, here we paid our attention to need for developing of such processes, which would have been used for commercial, large-scale manufacturing with minimum harm for environment and would have been different for their safety while contacting the workers and for high efficiency as well. A few synthesis methods of perfluoroalkyl ethylene derivatives are known. We will point some of them: pyrolysis of acetates of poly-fluorinated alcohols at 530-540 oC [7], dehydration of secondary and tertiary fluorine alcohols by dimethylsulphate [8], phosphoric acid [9], phosphoric anhydride [11], mixture of concentrated sulphuric acid and thionyl chloride [11]. These dehydrating agents are used in case of fluorinated diols for synthesis of unsaturated fluorine alcohols [12]. Main disadvantages of these methods are: synthesis duration, high temperatures, multi-stage process, using of toxic dehydrating agents, forming of highly toxic compounds and unsatisfactory purity of target products. Studying fluorine alcohols dehydration by P2O5 brought us unsatisfactory results. Yield of fluoroalkylolefines proved to be low. This may be connected with simultaneous going of fluorine alcohols and P2O5 interaction process resulting in forming of (as showed in the work [13]) fluorinated dialkylpyrophosphates. We applied available, safe to handle, non-toxic zeolites: "NaA", "NaX", "CaA" etc. (see preliminary report [15]) to increase the efficiency of secondary and tertiary alcohols dehydration (obtained according to [14]) using concentrated sulphuric acid. Thus, influence of concentrated sulphuric acid on 3,3,4,4-tetrafluorine-2-methylbutan-2-ol (1b) in the presence of zeolites results in its dehydration at 50-55 oC, that leads to forming of 3,3,4,4-tetrafluorine -2-methylbut-1-ene-3 2b of literally quantitive yield at content of main component equal to 99,9 %. 3,3,4,5,5,5-hexafluorine-2-methylpent-1-en (2d) was obtained the same way out of 3,3,4,5,5,5-hexafluoro-2-methylpentan-2-ol (1d). As we can see by the data of table 1 the addition of sulphuric acid in amount no less than 0.5 mole results in significant lowering of target products purity (column 8), and its use in amount more than 1 mole is not rational in terms of economics, because the increasing of target products purity doesn't take place (column 9). The alteration of amount of zeolites taking part in synthesis beyond the range of 1-40% of alcohol involved doesn't provoke the increase of target products yield (columns 6, 7). An optimal rate for carrying out a process of perfluoroalkyl containing olefins had been found in a temperature range of 40-120 oC, at that the lowest limit corresponds to reaction's start temperature, and exceeding the highest one doesn't positively influence the rate of reaction (column 5). The optimal proportion for sulphuric acid : polyfluorinated alcohol is a 0.5-1 : 0.3-0.5 ratio. Table 1.Fluorocontaining alcohols dehydration in H2SO4 at zeolite presence
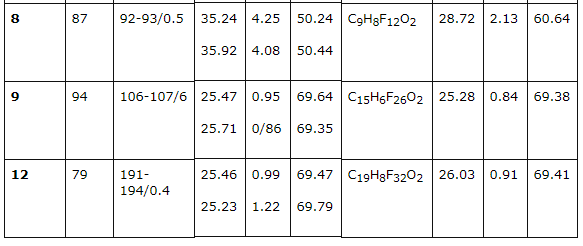
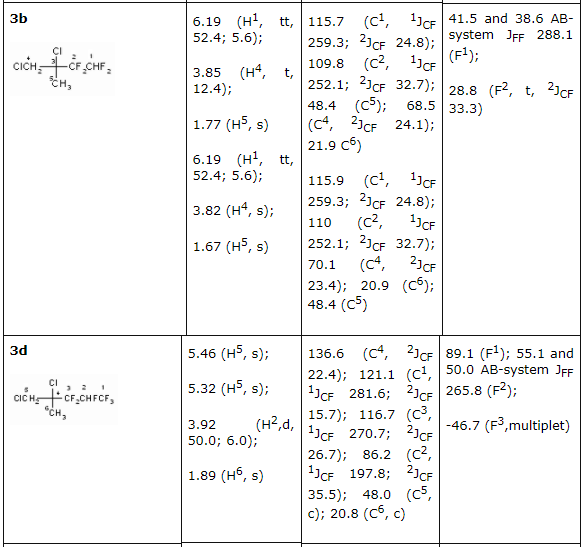
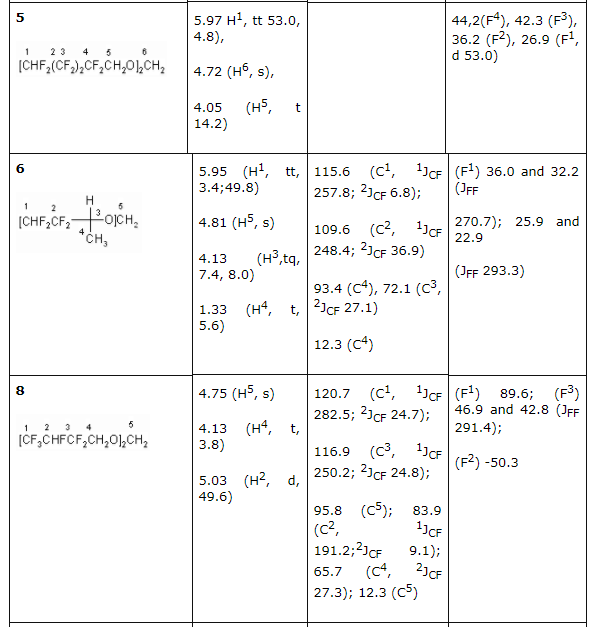
2b and 2d olefines' chlorination was carried out according to the method [16] at 25 - 30 oC in a solution of concentrated hydrochloric acid (chlorine was obtained at temperature of 20 oC at hydrogen peroxide slow adding) upon gas isolation stopping. Under these conditions an addition of chlorine is taking place according to multiply bond forming chlorinated hydrocarbons (R)-3,4-dichloro-1,1,2,2-tetrafluoro-3-methylbutane 3b and (4R)-4,5-dichlorinehexafluorine-4-methylpentane 3d respectively. We should note, that compound 3d is a mixture of diasteroisomers of ratio equal to 3:4. Both 3,4-dichlorine-3(chloromethyl)-1,1,2,2-tetrafluorobutane 4b and 4,5-dichloro-4(chloromethyl)-hexafluoropentane 4d form as addmixtures respectively. Compound 4d is also obtained in the form of diasteroisomers' mixture. These compounds can be of interest as anesthetics. Earlier [17] we had shown a forming of bis(octafluoropentyloxy)methane [acetal (5)] at interaction of telomeric alcohol H(CF2CF2)2CH2OH and CH2O in the presence of concentrated sulphuric acid. Later this observation was used to obtain polymers based on interaction of perfluoropolyethers diols (molecular mass in the range of 1000-2000) and formaldehyde in sulphuric acid [18]. Such compounds found their application as plasticizers for fluororubber for them to become durable, highly frost and aggressive resistant. Here we have stated, that primary and secondary fluorinated alcohols react with formaldehyde in the medium of concentrated sulphuric acid forming acetales. At that linear fluorine alcohols as well as telomeric alcohols produce acetales with high yield. We obtained bis(2,2,3,4,4,4-hexafluorobutoxi)methane (8) by interacting 2,2,3,4,4,4-hexafluorobutan-1-ol and formaldehyde in solution of sulphuric acid, and acetal (6) in the form of diastereoisomers' mixture was obtained by interacting secondary alcohol CHF2CF2CH(CH3)OH and formaldehyde. Increasing the length of hydrocarbonic chain of alcohol results in lowering of target product yield, especially it is noticeable for H(CF2CF2)4CH2OH alcohol. We recommend to carry out the process within the T range of 60-70 oC at mixing and 5-6 hours ageing for its increase.
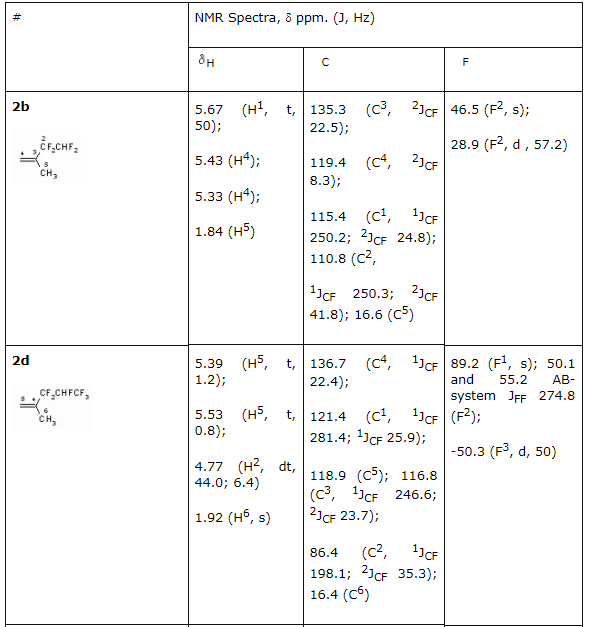
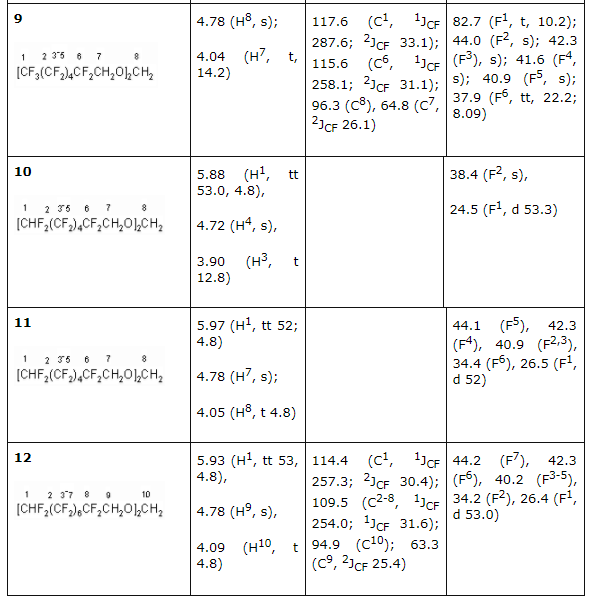
There had been no success in introducing the tertiary alcohols: CHF2CF2C(CH3)2 and CF3CHFCF2C(CH3)2OH into the reaction with formaldehyde in the medium of sulphuric acid. This must be caused by the easy dehydration of these alcohols, which leads to olefins formation. For the purpose of obtaining high purity acetales we had carried out the optimization of process conditions. As acetales are only partly soluble in concentrated sulphuric acid an opportunity to isolate them from acidic solution arose, from the one which had been returned to repeat the process. This greatly reduces the environmental risk of the present technology. The structure of obtained products 2-12 is confirmed by spectroscopy data of NMR, which was construed taking into account the data obtained before for the compounds of such type (Table 2) and mass-spectroscopy. In NMR 1H spectra of synthesized partly fluorinated compounds the signals of protons of -CH2- and CH3 substituents are seen, which chemical shifts values are typical for hydrocarbon analogues of such kind (Table 2). In NMR 19F and 13C spectra we can observe signals from fluorine and carbon atoms, which are typical for hydrocarbon skeletons, at that for fluorine atoms of CF2 groups, located at dyssimetric carbon atoms the signals' structure is an AB-system with JFF equal to 273-305 Hz. Fluorine atoms located at CHF and CHF2 (Table. 2) are experiencing main changes. Table2. NMR 1H, 13C, 19F spectra of new partly fluorinated compounds
   Obtained acetales can be effective heat-transfers and their final fluorination by elemental fluorine brings up the opportunity of obtaining the dielectrics having perspectives of variable boiling point. Experimental Part NMR 1H, 13C, 19F spectra were obtained using the spectrometer of Bruker WP 400 SY (400, 100, 188 MHz respectively), internal standard HMDS, C6F6 ( JCH not measured). IR-spectra were recorded using spectrometer of Specord M-80 (CCl4); Chromato-mass-spectra ( 70 eV ) were recorded using chromatographer with mass-selective detector (Hewlett Packard G 1800 A GCD) (we used column of 30m, 0.25mm diam., plated inside with 0.25 micrometer layer of co-polymer of 5% diphenyl and 95% dimethylsiloxane (HP-5), gas-transfer - helium, 1 ml/min-1, eveporater temperature was 280 oC. Column temperature was being increased at a rate of 10 degrees/min -1 starting from 50 (was kept for 2 min) up to 280 oC (was hold for 5 min). All the reactions were controlled using method of NMR 19F. The analysis of reaction mixtures was carried out at chromatograph of LHM 72 (15 % SE-30, CCTF-803, QF-1, chromosorb - W, column of 4000 mm, 4 mm diam.). Characteristics for new compounds and their analytical data are listed in tables 2,3. Table 3.Characteristic of partly fluorinated compounds and analytical data
Dehydration of 2,2,3,3-tetrafluoro-1-methylbutyl-2 alcohol All the synthesis were carried out using set containing of three-neck flask of 5l volume, equipped backflow condenser, and target product extarction system working while synthesis is underway. 170 ml of concentrated suplhuric acid, 50g of 2,2,3,3-terafluoro-1-methylbutyl-2 alcohol and 20g of ceolites were put into reactor, then heated up to 50-55oC and after that the extraction of 2b target product into cooled collector was started. Synthesis period was within 2-30 minutes. As a result we got 25-30g of 2b 1,1,2,2-tetrafluoro-3-methylbutene-3 of 99.8-99.9 % (vol.) purity. The product doesn't require additional purification. B.p. is 52-53 oC/740 mm [according to data [8] - b.p. is 51.5 oC/758 mm). Ananlogously we obtained compounds 2a,c,d (Table 2,3). Chlorinating Techniques for Polyfluorinated Olefines 1 mole of polyfluorinated olefine and 230g of hydrochloric acid (concentration no less than 35%) are put into round-bottom, three-neck flask, equipped with stirrer, effective backflow condenser (with flask filled with 10% KOH aqueous solution at outlet) and drop funnel. The flask is put into water bath and then at temperature no higher than 20 oC they slowly, constantly stirring add dropwise a 120g of hydrogen peroxide through drop funnel, after that they raise temperature up to 25-30 oC. They judge on reaction finish by stopping of gas isolation. After carrying out the synthesis the reaction mass is put out, organic layer is isolated (using separating funnel), then the reaction mass is weighted and analyzed at chromatographer (see Table 2,3). General Synthesis Technique for Acetales Based on Telomeric Alcohols and Formaldehyde 200 ml of concentrated sulphuric acid is poured into three-neck flask of 0.5l volume, equipped with stirrer, then the stirring is started, 15g of paraform is put into it and 132g of 2,2,3,3-tetrafluoropropyl alcohol is added. The flask is put into cold water bath to prevent heating and to keep temperature at 20 oC, it is stored at room temperature and constant stirring for 3 hours, then sulphuric acid is isolated at separating funnel and poured onto ice, small in weight organic layer is being isolated, combined with main organic mass, afterwards all that is dried with CaCl2 and fractionated (it's better to handle distillation at reduced pressure). The concentration of main component in target fraction counts to 93-95 % vol. at that. Compound 10 is obtained, b.p. is 62-63 oC/18 mm Hg [according to data [17] b.p. 57-58 oC/ 5 mm Hg.]. Sulphuric acid may not be diluted with water and you may use it again for next experiment or to accumulating a target product. Characteristics of compounds 5, 11, 12 had matched with data, displayed for that compounds in the work [17]. Compounds 5-9 were obtained ananlogously (see Table 2,3). Conclusions 1. Partly fluorinated alcohols undergo dehydration with further forming of ethylene and propylene derivatives when subject to sulphuric acid at a presence of zeolites. 2. Fluoroalcohols and formaldehyde reaction in the medium of concentrated sulphuric acid results in forming of acetales, which can be used as high-temperature heat-transfers and their additional fluorination results in forming of fully fluorinated dialkyl ethers which are of interst as effective dielectrics with wide range of boiling points. REFERENCES [1] Organofluorine Chemistry, Principles and Commercial Applications. / Eds/ Banks R.E., Smart B.E., Tatlow J.C. Plenum Press : New York. 1994. 287 P. [2] Uklonsky I.P., Denisenkov V.F., Ilyin A.N. // Pat. 2150459 Russia (16.06.2000). CI. C 07 C 31/38. Sposob poluchenya polyftorirovanych alcohols.; Chem. Abstr. 2002. v. 136. 218614b. [3] Uklonsky I.P., Denisenkov V.F., Ilyin A.N. // Pat. 2150459 Russia (27.07.2003). CI. C 07 C 31/38. Sposob poluchenya polyftorirovanych alcohols.; Russ. Abstracts. 05.09 - 19H.99P. [4] Tohma T., Wada A. // Pat. 6861565 U.S. (01.03.2005). C 07 C 31/34. Process for producing a fluoroalkanol. ; Russ. Abstracts. 05.24 - 19H.159P. [5] Furin G.G., Ilyin A.A., Ivanova L.M. et al. // Nauka proizvodstvu. 2004. v. 5. p. 26-36. [6] Furin G.G., Ilyin A.A., Ivanova L.M. et al. // Khimia v interesach ustoichivogo razvitya. 2005. v. 13. N. 6. p. 831-838. [7] Rondestvedt C.S. // J. Org. Chem. 1977. v. 42. N. 15. p. 2618-2620. [8] No V.B., Bakhmutov Yu.L., Martinova N.P. et al. // Patent USSR 480692 (30.09.1976). CI. C 07 C 21/18. Sposob poluchenya ftorsoderzhashich olephinov.; Russ. Abstrs. 1977, 13 H 7P. [9] Methods eksperimtnt in organicheskoy khimii // Ed. N.I. Syvorov. M. : Khimia. 1968. [10] Frisch E.E., Steward O.W. // Pat. 3202643 U.S. (24.08.1965). CI. 260-92.1. 3,4,5,5,5-penta-fluoropentadiene and a homopolymer thereof.; Russ. Abstrs. 1967. 5 C 209P. [11] Bakhmutov Yu.L., Belogay V.D., Charchenko A.P. et al. // Patent USSR 635083 (19.04. 1977). CI. C 07 C 21/18; C 07 C 17/24. Sposov polychenya polyftorirovanich olephins..; Bul. Izobret. 1978. v 44. p. 88. [12] Lichstein B.M., Woolf C. // Pat. 3576888 U.S. (27.04.1971). C 07 C 33/10/ Process for the preparation of unsaturated fluorinated alcohols.; [13] Sadych S.I., Guseinov M.M., Ashurov D.A. et al. // Patent USSR 375923 (02.08.1973). CI. C 07 C 19/02; C 07 C 23/10; C 07 C 25/14. Sposob polychenya 1,2,3-trichlorpropane or 1-phenyl-1,2-dichlorethane or 1,2-dichlorocyclohexane.; Russ. Abstracs. 1974. 6H10P. [14] Ilyin A.A., Bokhmutov Yu.L., Ilyin A.N. et al. // Zh. Prikl. Khim., 2004. v. 77. N. 1. p. 102-105. [15] Ilyin A.A., Denisenkov V.F., Ilyin A.N. et al. // Pat. 2245319 Russia (27.01.2005). CI. C 07 C 21/18. Sposob polychenya ftorsoderzhashich olephinov.; Russ. Absytacts. 05.09 - 19H.100P. [16]. Fedoseev M.S., Minyailo V.V., Il`yn A.N. et al. // Patent USSR 1469819 (1987), CI. C 07 F 9/11, C 08 C 18/16. [17] Hill M.E., Shipp K.G. / Pat. 3526667 U.S. (1.09.1970). C 07 C 43/00. Process for acetal preparation. [18] Scicchitano M., Turri S. J. Fluorine Chem. 1999. v. 95. p. 97-103. |
Fluorine Notes, 2007, 50, 1-2