Fluorine Notes, 2006, 46, 1-2
PRESENT-DAY CONDITION OF FLUOROAROMATIC COMPOUNDS PRODUCTION TECHNOLOGYG.G. Furin a *, L.E. Deev b a* Novosibirsk Institute of Organic Chemistry , Russian Academy of
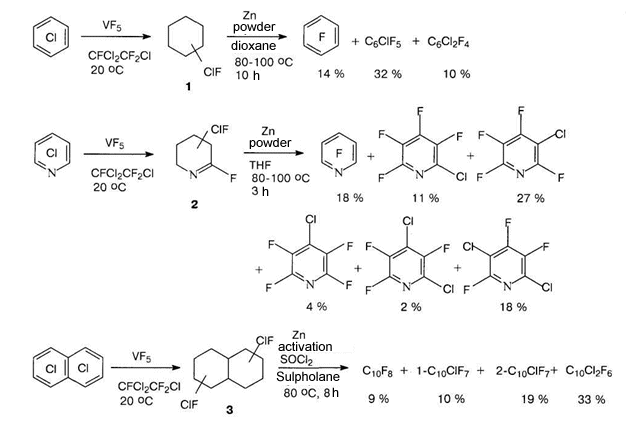
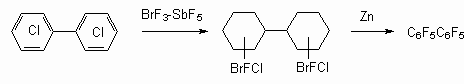
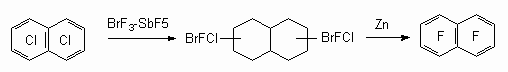
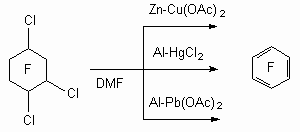
Science 630090, Novosibirsk, Ac. Lavrentiev av. 9, b Perm State Agricultural Academy, 614990, Perm, e-mail : deev@permonline.ru Here we describe effectiveness of interphase transfer catalysts use to obtain polyfluoroaromatic compounds by potassium fluoride influence on polychlorbenzenes. Such catalysts as hexaethylguanidine chloride, tetra-(diethylamino)- phosphonium bromide are involved into stabilization of intermediate s-complex. Catalytic participation of polyethers (tetraethyleneglycole dimethyl ether, 18-crown-6) in fluorodechlorinating process doesn't go beyond increasing of "active" fluoride-ion concentration. Here we consider the opportunities of mechanic and chemical technology application to synthesize fluoroaromatic compounds by substituting chlorine for fluorine in the solid phase of chloroaromatic compounds and fluorides of alkali, alkali-landed metals and composite mixtures based on them. We also discuss the question regarding synthesizing fluoroaromatic compounds out of commercial chladones (freons) and polyfluorolefines. Contents Introduction 1. Hexafluorobenzene synthesis by potassium fluoride influencing hexachlorobenzene in the presence of catalysts. 2. Mechano-chemical obtaining method of hexafluorobenzene. 3. The using of polyhaloidbenzenes fluorination and fluorination products' dehalogenation processes as obtaining method of hexafluorobenzene and other aromatic compounds. 4. Synthesis of fluoroaromatic compounds out of commercial chladones and polyfluorolefines. Conclusion References Polyfluoroaromatic compounds obtaining method was worked out, a process of halogens removal in cyclic alkanes had formed its base. For example, initially fluorine containing cycloalkane C6BrCl6F5 is obtained out of hexachlorobenzene under the influence of BrF3-SbF5this cycloalkane is turned into hexafluorobenzene [76] under the influence of zinc powder in ethyl alcohol. In the work [77] hexachlorohexafluorocyclohexane 1 is obtained with quantitive yield by fluorinating of hexafluorobenzene VF5 in chlorofluorocarbons (CFCl3, CFCl2CF2Cl) at 20-50 oC. Analogously tetrachlorpentafluoro-1-azacyclohexenes 2 and octachlorooctafluorobicyclo[4.4.0]-decenes-1(6) 3 were obtained out of pentachloropyridine and octachloronaphtalene respectively. Dechlorination of these compounds using zinc in different status (zinc activated complex NiCl2*6H2O*2,2'-dipyridil; treated with SOCl2; powder; zinc-copper pair) results in forming of mixture containing hexafluorobenzene, chloropentafluorobenzene and dichlorotetrafluorobenzene [78]. Both solvent origin and zinc condition [78] influence the yield of dechlorination products. It should be noted, that other reagents are effective for this process as well.
For example, at tris(diethylamino)phosphine P(NEt2)3 influencing onto 1 and 3 compounds the mixtures of fluorobenzenes and naphthalene with different number of fluorine atoms were obtained[78].
The method is rather effective and it was used to obtain other polyfluoroaromatic compounds: decafluorobiphenyl [79,80], octafluoronaphtalene and others.
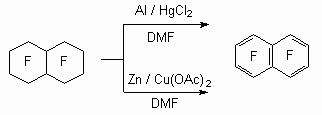
Zinc-copper pair can act as dechlorinating agent in dimethylformamide (DMF) or dimethylacetamide. Thus, octafluoronaphtalene of good yield [81] and hexafluorobenzene were obtained.
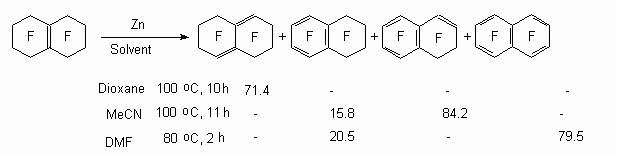
It should be noted, that zinc as itself is not effective, here we need a solvent [82]. It is shown using an example of hexadecafluorobicyclo[4.4.0]dec-1(6)-ene.
The aromatization process of perfluorocyclohexene is going analogously.
Poly-chlorofluorocycloalkanes are being dehalogenated using M/M2+ metals system, where M = Zn, Al; and M2+ = Cu2+, Hg2+, Sn2+, Pb2+ . The yield of hexafluorobenzene and octafluoronaphtalene amounts to 70 - 80 %.
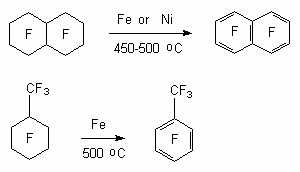
One of the approaches to synthesis of polyfluoroaromatic compounds is a transforming of saturated cyclic perfluorocarbones under the influence of nickel, iron at 400-600oC [83,84]. This approach had been developed in 60-s. However, because of low yield of target products and unavailability of original substrates it had not found a wide spread occurrence and commercial application. At the same time it showed a fundamental opportunity of implementation of such approach to synthesis of poly-fluoroaromatic and poly-fluoroheterocyclic compounds.
Dechlorinating by iron powder at 500 oC was carried out for chlorine containing cyclic alkenes, at that as a rule there is being formed a mixture of products [78].
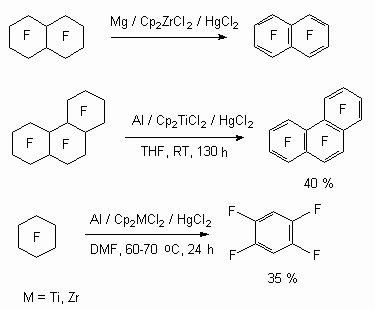
Along with development of direct fluorinating methods of aromatic compounds and cyclic carbons a raw materials base had appeared for realization of defluorination and dechlorination of compounds of such type. They use not only metals of variable valency as reagents for this process in the presence of catalysts but also anion-radicals of some organic compounds [85]. Thus, Mg, Al metals in the presence of Cp2MCl2 (M = Ti, Zr)-HgCl2 system, acting as catalyst, carry out defluorinating of perfluorinated cyclic compounds at room temperature forming polyfluoroaromatic compounds [85].
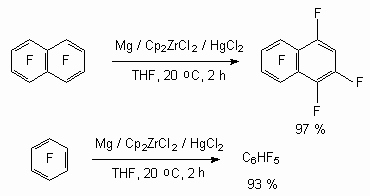
It should be noted, that in case of perfluorocyclohexane C-F bond hydrogenolysis must occur in hexafluorobenzene, forming during this process under the influence of reagents. Indeed it was shown[86], that present system of titanocene and zirconocene complexes catalyses reduction process of polyfluoroaromatic compounds under the influence of magnesium in the tetrahydrofurane solution. Thus, heptafluoronaphtalene and pentafluorobenzene are formed out of octafluoronaphtalene and hexafluorobenzene respectively.
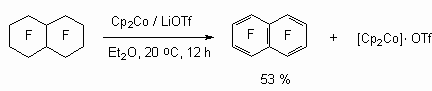
It was shown [87], that acting of stochiometric quantities of cobaltocene in the presence of LiO3SCF3 at its influence on perfluorodecalin results in forming of octafluoronaphtalene.
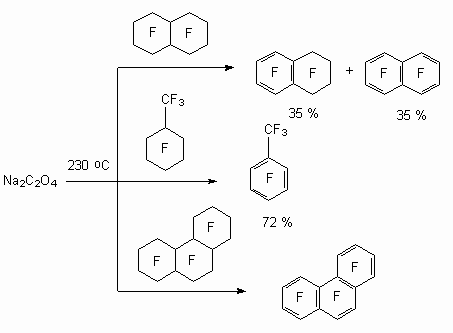
[( Thermal method was also used for these purposes. Thus, influencing of sodium oxalate onto perfluorodecalin or perfluoromethylcyclohexane at heating up to 230oC octafluoronaphtalene and octafluorotoluene were obtained respectively [88-91].
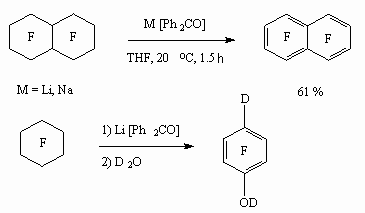
Perfluorocarbons high selective reduction process is implemented using an example of benzophenonee anion-radical influencing perfluorodecalin and perfluorocyclohexane in terahydrofuran [89-91].
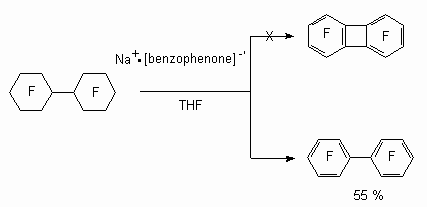
In case of perfluorocyclohexane there is a formation of low yield of terafluorophenols. At the same time when benzophenone anion-radical influencing perfluoro(bicyclohexyl) reduction defluorination goes at high selectivity at low temperature forming decafluorodiphenyl but not octafluorodibenzobutane [92].
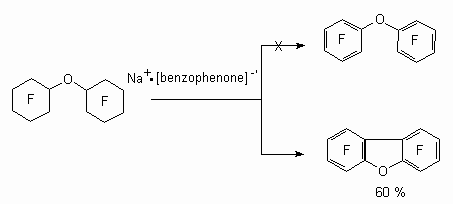
At the same time the reductive defluorination of perfluorobicyclohexyl ester is followed not by forming of perfluorodiphenyl ester, but by forming of perfluorodibenzofuran [92].
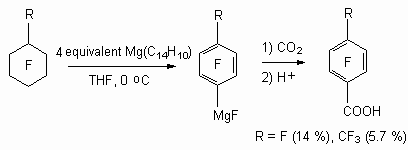
Perfluorocyclohexane and perfluoromethylcyclohexanen were exposed of reductive defluorination at action of antracene mono-anion MgC14H10 in tetrahydrofuran at -41 oC producing corresponding Grignard reactive. Further they reacted with carbon dioxide producing pentafluorobenzoic and 4-trifluoromethyl-2,3,5,6-tetrafluorobenzoic acids correspondingly [93].
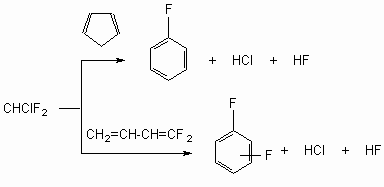
The alternative strategy of fluoroaromatic synthesis lies in synthetic production of assigned fluorobenzene-like structures with using reactive fluorine containing fragments (fluorosynthones). The application opportunities for this methods for synthesis of mono-, di-, tri- and polyfluorosubstituted arenas are based on application of commercial chladones (freons) and poly-fluoroolefines as feedstock or fluorine containing structure blocks [94,95]. CHCl3, CHClF2, CHCl2F, CClF3, CF2=CF2, CF3CF=CF2, CF2CFCl, CF2=CFC4F9, CF3CF=CFC3F7etc. are used as such ones [18]. This approach opens large synthetic perspectives for introducing fluorine into aromatic nucleus. It is based at gas-phase generating and cyclic addition of fluorocarbenes and poly-fluorolefines to unsaturated hydrocarbons followed by thermal isomerization of fluorine containing cyclopropanes and cyclobutanes. Butadiene and its methyl derivatives, cyclopentadiene etc are used as such compounds. Obtaining method of fluorine aromatic compounds using gas-phase reaction of difluorocarbene and coupled dienes was implemented in the form of universal mono- and difluorobenzenes obtaining technology. The technology lies in gas-phase copyrolysis of difluorochlomethane (chladone 22) and cyclopentadiene (fluorobenzene obtaining [96-99]) either by butadiene -1,3 (obtaining of difluorobenzenes [97-100]). The process is carried out in flow system on a continuous mode with water vapour, water-ammonia mixtures [95] or at alkaline packing [87], linking isolating halogen hydrogens, what increases selectivity of synthesis and lowers the resinification [18].
Carbenes method was successfully used to synthesize the fluorine containing bicyclic aromatic compounds: 2-fluoronaphthalene (yield is 68-80 %), starting from indan and CHClF2 at 600-670oC [101], 2,3-difluoronaphthalene (yield is 16-65 %), starting from styrene and CHClF2 at 650 oC [102,103]. Obtaining method of 1,2-difluorobenzene, 3,4-difluorotoluol, 2,3- difluorotoluol and 1-fluoro-2-trifluoromethylbenzene is based on pyrolysis of substituted derivatives of vinylcyclobutane of the type listed below at 600-800 oC in the presence of water vapour [104]. Thus, 2,3-difluoro-2,3-dichlorocylobutane produces ortho-difluorobenzene after water steaming and ammonia treatment, and 1-vinyl-2,3-difluorocyclobutane transforms into 1,2-difluorobenzene with the yield of 61.5 % (conversion of starting one is 80 %) [104].
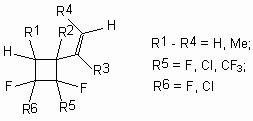
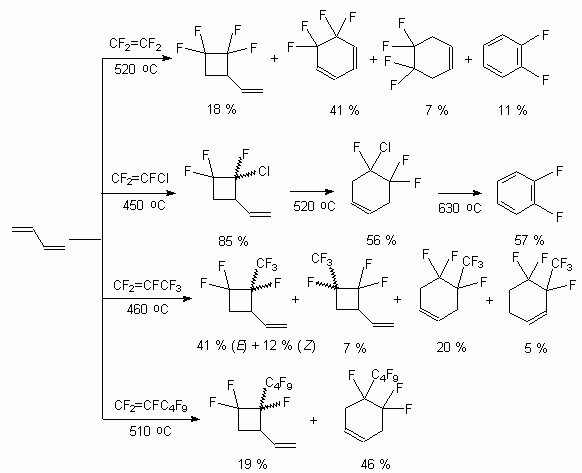
Fluorolefines can thermally add 1,3-dienes in a mode of [4+2] and [2+2]- cycloaddition [105]. Fluorinated cyclohexene and vinylbutane adducts formed during that processes can be transformed into partly fluorinated aromatic compounds either directly by the following dehydrohalogenation or through the preliminary stage of vinylcyclobutane-cyclohexene rearrangement [106,107]. This approach was used for working out the obtaining method of partly fluorinated benzene derivatives using available fluorolefines. It lies in constructing fluorobenzene-like structures by fluorolefines thermal cyclic addition of 1,3-diens and further aromatization of forming fluorinated carboxylic adducts. Thus, interaction of tetrafluoroethylene and 1,3-butadiene in a flow reactor results either in forming of 1-vinyl-2,2,3,3-tetrafluorocyclobutane (temperature was 450-470 oC, contacting period 4-6 seconds) with the yield within 78-85% or in forming of fluorocyclohexane (temperature 490-520oC) [106]. The reaction has a common nature and trifluorochloroethylene, hexafluoropropylene, perfluoropropylene, perfluorohex-1en, perfluorohex-2en are introduced into it.
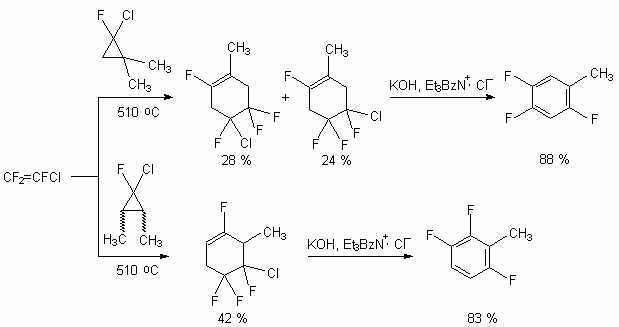
Not only derivatives of 1,3-butadiene (2-methyl-1,3-butadiene, piperylene, 2,3-dimethyl-1,3-butadiene, haloisoprene derivatives) but also hem-fluorochlorocyclopropane are introduced into the reaction [106]. For example, the interactrion of trifluorochloroethylene and 1,1-dimethyl-2-fluoro-2-chlorocyclopropane results in forming of isomeric methyltetrafluorocyclohexenes as main products, which not being separated are transformed into 2,4,5-trifluorotolyol by alkali dehydrohalogenation under the conditions of inter-phase catalysis [106].
Thus, new methods of fluoroaromatic compounds synthesis using the reactions of thermal transformation of poly-fluoroolefines allows to offer alternative to traditional methods of solving a problem of obtaining low fluorinatinated benzene derivatives not only for preparative purposes but also for commercial production. The aims of present review are analyzing of new file of information regarding reactivity of fluoroaromatic compounds, which was accumulated during the last decade, describing of fluorine atoms introduction's into benzene ring influence on properties of some benzene derivatives, also describing of development of new methods of fluorine containing aromatic compounds synthesis, latest achievements of this class of compounds. Here we also targeted on presenting the information on practical using of fluorinated aromatic compounds. In our opinion, it can forward a wider attracting of poly-fluorinated organic compounds to solve a number of crucial questions of theoretical organic chemistry and also to purposefully synthesize compounds, possessing useful properties. 1. Vorozhtsov N.N., Platonov V.E., Yakobson G.G. Izv. Akad. Nauk SSSR. Ser. Khim., 1963, N 8, p. 1524; Pat. 166661 SSSR.BI 1964. N 23. p. 17. 2. Vorozhtsov N.N., Zh. Vses. Ob-va im D.I. Mendeleeva. 1970, v. 15, N 1, p. 52-63. 3. Yakobson G.G., Shteingarz V.D., Vorozhtsov N.N. Izv. Akad. Nauk SSSR. Ser. Khim., 1964, N 8, p. 1551; Pat. 162826 SSSR. BI 1964. N 11. p. 14. 4. Coe P.L., Stuart A.M., Moody D.J. J. Chem. Soc., Perkin Trans, 1, 1998. p. 1807-1811. 5. Chambers R.D., Skinner C.J., Hutchinson J., Thomson J. J. Chem. Soc., Perkin Trans,1, 1996, p. 605-609. 6. Furin G.G., Fainzilberg A.A. Modern methods for fluorination of organic compounds. M. : Nauka. 2000. 239 P. 7. Chem. Eng. (USA), 2002, v. 109, N 11, P. 19; Russ. Ref. Zh. 03.22-19N.10. 8. Subramanian M.A. Pat. 6166273 U.S. (2000); Chem. Abstr., 2001, v. 134, 41972; Russ Ref. Zh. 02.05-19N.81P. 9. Amii H. Kagaku. 2003. v. 58. N. 2. P. 46-47; Chem. Abstr., 2003, v. 139, 135150. 10. Chambers R.D., Hutchinson J., Murgrave W.K.R. J. Chem. Soc., 1964, p. 3573; 11. Banks R.E., Haszeldine R.N., Latham J.V., Young I.M. Chem. Ind., 1964, p. 835. 12. Hirota K. (Nippon Shokubai Co., Ltd., Japan) Ger. Offen. DE 10143395 (2002); Chem. Abstr., 2003, v. 136, 233888. 13.Chambers R.D., Hole M., Iddon B., Musgrave W.K.R., Storey R.A. J. Chem. Soc. (C), 1966, p. 2328 14. Reactionen sposobnost polyfluoroaromatic compounds. Ed. G.G. Yakobson. Novosibirsk : Nauka. 1983. 250 P. 15.Clark J.H., Wails D., Bastock T.W. in : Aromatic fluorination. CRC Press. 1996, 186p. p.19-55. 16. Fluorination of Aromatic Compounds by Halogen Exchange with Fluorine Anions (?Halex? Reaction) Eds. Desmurs J.-R., Ratton S. L. : Elsevier, 1996, p. 244-275. 17. Chemistry of Organic Fluorine Compound II. Eds. Hudlicky M., Pavlath A.E. ACS Monograph 187. American Chemical Society. Washington DC. 1995. 18. Nefedov O.M., Volchkov N.V. Zh. Org. Khim., 1994, v. 30, N. 8, p. 1123-1135; Chem. Abstr., 1995, v. 123, 198324h. 19. Brooke G.M. J. Fluorine Chem., 1997, v. 86, p. 1-76. 20. Fuller G. J. Chem. Soc., 1965, p. 6264 21. Chambers R.D., Hole M., Iddon B., Musgrave W.K.R., Storey R.A. J. Chem. Soc., 1966, p. 2328. 22. Kiplinger J.L., Richmond T.G. J. Am. Chem. Soc., 1996, v. 118, p. 1805-1806. 23. Kaieda O., Hirota K. Pat. 6392084 U.S. (2002); Russ. Ref. Zh. 03.15-19N 101P. 24. Hirota K. Pat. 6437168 U.S. (2002); Russ. Ref. Zh. 03.10-19N 89P. 25. Takaoka A., Yokokohji O., Yamaguchi Y., Isono T., Motoyoshi M., Ishikawa N. Nippon Kagaku Kaishi. 1985. p. 2155; Chem. Abstr., 1986, v. 105, 152649t. 26. Ishikawa N., Kitazume T., Yamazaki Y. Chem. Lett. 1981. p. 761-766. 27. Clark J.H., Hyde A.J., Smith D.K. J. Chem. Soc., Chem. Commun., 1986, p. 791. 28. Smyth T.P., Carey A., Hodnett B.K. Tetrahedron, 1995, v. 51, N 22, p. 6363-6368. 29. Kimura J., Suzuki H. Tetrahedron Lett., 1989, p. 1271-1273. 30. Shipilov A.I., Fedoseev N.G. Zh. Prikl. Khim., 2000, v. 73, N. 3, p. 522-523; Chem. Abstr., 2000, v. 133, 19070j. 31. Ishikawa N., Kobayashi Y. Fluorine compounds. Chemistry and Application. Ed. Ishikawa N. M. : Mir. 1990. 405 P. 32. Allison K.R. Pat. 4069262 U.S. (1978); Russ. Ref. Zh., 1978, 21 O 542P. 33. Kimura Y. ,1989, v. 47, N 8, p. 258-264. 34.Brooke G.M. J. Fluorine Chem., 1997, v. 86, N 1, p. 1-76. 35. Demlov E., Demlov Z. In Mezhfaznii catalysis in organic chemistry. M. : Mir. 1980. 327 P. 36. Veber V., Gokel G. In Mezhfaznii catalysis in organic chemistry. M. : Mir. 1980. 327 P. 37. Yanovskay L.A. Izv. SO Akad. Nauk CCCH. Ser. Khim. N., 1983, Vip. 4, N 9, p. 11-20. 38. Shen Z.-Q., Chen J.-H., Jin W.-Q., Gu L.Q. Fine Chem. 2001, v. 18, N 12, p. 713-714; Russ Ref. Zh. 02.13-19N.82. 39. Murray C.B., Sandford G., Korn S.R. J. Fluorine Chem., 2003, v. 123, p. 81-84. 40. Sasson Y., Negussie S., Royz M., Mushkin N. J. Chem. Soc., Chem. Commun., 1996, p. 297-298. 41. Deev L.E., Nazarenko T.I., Podsevalov P.V. Zh. Org. Khim., 1994, v. 30, N. 12, p. 1829-1832. 42.Schach Th., Wessel Th., Gutermuth M. Pat. 19745212 Germany, (1999); Russ. Ref. Zh. 00.12-19N. 149P. 43. Schach T., Papenfuhs T., Kaus A., Gutermuth M. Pat. 19953194 Germany (2001). Russ. Ref. Zh. 02.08-19N.85P. 44. Miyajima F., Iijima T., Tomoi M., Kimura Y. React. Fuct. Polym., 2000, v. 43, N 3, p. 315-324; Chem. Abstr., 2000, v. 132, 307941r. 45. Clark J.H., Macquarrie D.J. Tetrahedron Lett., 1987, v. 28, N 1, p. 111-114. 46.Kumai S. // The seventh regular meeting of Japanese-Soviet Fluorine Chemists. Symposium on Fluorine Chemistry . May 15-16. 1991. Fukuoka. Japan. Abstracts Lecture. 13.1 - 13.10. 47. Brown S.J., Clark J.H. J. Fluorine Chem., 1985, v. 30, p. 251-258. 48. Kusov S.Z., Lubenez E.G., Kobrin V.S., Chmelnitzkii A.G. Izv. SO Akad. Nauk SSSR. Ser. Khim. N., 1986, Vip.. 3, p. 81. 49. Berris B., Cheng C. Pat. U.S. 6046358 (1999). 50. Bildinov I., Podsevalov P., Nazarenko T., Deev L., Owens D.W., Balhoff J.F. EP Pat. 0944564 (1998). 51. Kolomeitsev A., Pasenok S. DE Pat. 19631854 (1998). 52. Schiemenz B., Wessel T., Pfirmann R. DE Pat. 19934595 (2001). 53. Henrich M., Marhold A., Kolomeitsev A., Roschenthaler G.-V. EP Pat. 1266904 (2002). 54. Schanen V., Cristau H.-J., Taillefer M. WO Pat. 092226 (2002). 55. Kolomeitzev A.A., Pasenok S.V., Marchenko A.P., Koidan G.V., Polydnenko V.G., Pinchuk A.M., Yagupolskii Yu. L., Yagupolskii L.M. Ukr. Khim. Zh., 2002, v. 68, N 5-6, p. 103-106; Chem. Abstr., 2002, v. 137, 369779f. 56. Wessel T., Decker D., Huenig H., Schwesinger R. (Clariant G.m.b.H., Germany) PCT Int. Appl. WO 2003 101926 (2003); Chem. Abstr., 2004, v. 140, 16651. 57. Pleschke A., Marhold A., Schneider M., Kolomeitsev A., Roschenthaler G.-V. J. Fluorine Chem., 2004, v. 125, p. 1031-1038. 58. Otto N., Norbert L. Pat. 19740632 Germany (1999); Russ. Ref. Zh. 00.11-19R. 120P. 59. Shipilov A.I., Bikova A.B., Elochova L.I., Igumnov S.M. Izv. Akad. Nauk. Ser. Khim., 2003, N 2, p. 464-468. 60. Berris D.C., Chemg C.-H. Pat. 5965781 U.S. (1999); Russ. Ref. Zh., 00.22-19N. 104P. 61. Zhu X.-D., Zhu X.-F. J. Qufu Norm. Univ. Natur Sci., 2001, v. 27, N 3, p. 61-64; Russ. Ref. Zh., 02.10-19N.124. 62. Bildinov I.K., Deev L.E., Nazarenko T.I., Podsevalov P.V. Pat. 2135453 Rossia (1999); BI N 24 (1999); Chem. Abstr., 2000, v. 133, 177010. 63. Igumnov S.M., Zabolotskikh A.V. Pat. 6265627 U.S. (2001); Chem. Abstr., 2001, v. 135, 124129z. 64. Igumnov S.M., Zabolotskikh A.V. Pat. 2164508 U.S. (2001); Russ. Ref. Zh. 01.14-19R. 92P. 65. Igumnov S.M., Shipigusev A.A., Nikulin K.B. Pat. 2157800 Russia (2000); Chem. Abstr., 2002, v. 136, 71530. 66. Owens D.W., Balhoff J.F. Pat. 6241917 U.S. (2001); Russ. Ref. Zh. 02.15-19N.113P. 67. Chem. and Eng. News., 1999, v. 77, N 43, p. 16; Russ. Ref. Zh. 00.08-19N. 147. 68. Wood A. Chem. Week., 1999, v. 161, N 40, p. 43; Russ. Ref. Zh. 00.16-19N. 5. 69. Aksenov V.V., Vlasov V.M., Maryakina I.M., Rodionov P.P., Fadeeva V.P., Chertok V.S., Yakobson G.G. J. Fluorine Chem., 1985, v. 28, N 1, p. 73-87. 70. Dushkin A.V., Karnatovskay L.M., Chubaeva E.N., Pavlov S.V., Kobrin V.S., Starichenko V.F., Kusov S.Z., Kniazev V.V., Boldirev V.V., Tolstikov G.A. Khim. In interress ustoichivogo razvitia. 2001, v. 9, N 5, p. 625-630. 71.Cacae F., Speranza M., Wolf A.P., Macgregor R.R. J. Fluorine Chem., 1982, v. 21, N 2, p. 145-158. 72. Shipilov A.I., Karjukalova N.N., Igumnov S.M. Zh. Org. Khim., 2002, v. 38, N. 2, p. 240-243. 73. Dushkin A.V., Kobrin V.S., Karnatovskay L.M., , Kniazev V.V.,Kobriva V.N., Kusov S.Z., Pavlov S.V., Grazhdannikov A.E., Chubaeva E.N., Boldirev V.V.,Matiucha V.A., Starichenko V.F., Tolstikov G.A., Chandorin G.P. Pat. 2176236 Russia (2001); Russ. Ref. Zh. 02.09-19?.96?; Pat. 2165404 Russia (2001). 74. Dushkin A.V., Karnatovskaia L.M., Chabueva E.N., Pavlov S.V., Kobrin V.S., Boldyrev V.V., Kobrina V.N., Grazhdannikov A.E., Knjazev V.V., Starichenko V.F. Synth. Commun., 2001, v. 31, N 7, p. 1041-1045; Russ. Ref. Zh. 02.08-19?.336. 75. Dushkin A.V., Karnatovskay L.M., ,Chubaeva E.N., Pavlov S.V., Kobrin V.S., Starichenko V.F., Boldirev V.V. Dokl. Akad. Nauk., 2000, v. 371, N 5, p. 632-633;. Chem. Abstr., 2000, v. 133, 179237x. 76.McBee E.T., Lindgren V.V., Ligett W.B. Ind. Eng. Chem., 1947, v. 39, p. 378-380. 77. Bardin V.V., Bardina S.G. J. Fluorine Chem., 2004, v. 125, p. 1411-1414. 78. Bardin V.V., Trukhin D.V., Adonin N.Yu., Starichenko V.F. J. Fluorine Chem., 2004, v. 125, p. 1431-1435. 79.McBee E.T., Lindgren V.V., Ligett W.B. Pat. 2488216 U.S. (1949); Chem. Abstr., 1980, v. 44, N 4, 1628. 80.McBee E.T., Ligett W.B., Lindgren V.V. Pat. 2589364 U.S. (1952); Chem. Abstr., 1952, v. 46, N 18, 8675d. 81. Igumnov S.N., Narinyan K.G., Igumnova E.V. Fluorine Notes, 1999, v. 1. 82.Hu C.-M., Long F., Xu Z.-Q. J. Fluorine Chem., 1990, v. 48, N 1, p. 29-35. 83. Gething B., Patrick C.R., Stacey M., Tatlow J.C. Nature, 1959, v. 183, p. 588-589. 84. Gething B., Patrick C.R., Tatlow J.C., Banks R.E., Barbour A.K., Tipping A.E. Nature, 1959, v. 183, p. 586-588. 85. Kiplinger J.L., Richmond T.G. J. Chem. Soc., Chem. Commun., 1996, p. 1115 86. Burdeniuc J., Jedlicka B., Crabtree R.H. Chem. Ber./ Recueil/ 1997, v. 130, N 2, p. 145-154. 87. Bennett B.K., Harrison R.G., Richmond T.G. J. Am. Chem. Soc., 1994, v. 116, p. 11165-11166. 88. Burdeniuc J., Crabtree R.H. Science, 1996, v. 271, p. 340-341. 89. Saunders G.C. Angew. Chem., 1996, Bd. 108, N. 22, s. 2783-2785. 90. Marsella J.A., Gilicinski A.G., Coughlin A.M., Pez G.P. J. Org. Chem., 1992, p. 2856; 91. Marsella J.A., Pez G.P., Coughlin A.M. Pat. 5026929 U.S. (1991). 92. Sung K.-S., Lagow R.J. J. Chem. Soc., Perkin Trans 1, 1998, p. 637-638. 93. Beck C.M., Park Y.-J., Crabtree R.H. J. Chem. Soc., Chem. Commun., 1998, p. 693-694. 94. Nefedov O.M., Ivashenko A.A. Novoe in chemistry karbene. M. : Nauka.1973, p. 145-149. 95. Chemistry of Carbenes and Shall-Sized Cyclic Compounds. Ed. O.M. Nefedov. M. : Mir 1989, p. 69-100. 96. Nefedov O.M., Ivashenko A.A. Pat. 277759 USSR (1970). 97. Nefedov O.M., Ivashenko A.A. Pat. 214530 USSR (1968). 98. Nefedov O.M., Ivashenko A.A. , Polytanskii S.F., Kushina I.V., Yakovlev V.M., Starichenko V.F. Pat. 572446 USSR (1977); BI N 34, (1977). 99. Kushina I.V., Polytanskii S.F., Shevchuk V.U., Gutor I.M., Ivashenko A.A. , Nefedov O.M., Izv. Akad. Nauk USSR. Ser. Khim., 1974, N 4, p. 946-949. 100. Nefedov O.M., Ivashenko A.A. Izv. Akad. Nauk USSR. Ser. Khim., 1968, N 6, p. 1417-1418. 101. Nefedov O.M., Ivashenko A.A. Pat. 279608 USSR (1967); BI N 27, 1970. 102. Nefedov O.M., Ivashenko A.A. Pat. 339535 USSR (1971); BI N 17, 1972. 103. Nefedov O.M., Ivashenko A.A. Pat. 290900 USSR (1970); BI N 3, 1971. 104. Kushina I.V., Guda V.M., Pozderskii Yu.A., Starichenko V.F., Rutkovskii E.K., Nefedov O.M. Pat. 43852 Ukraina (2002); Russ. Ref. Zh. 02.18-19N.86P; Pat. 2147569 Russia (2000); Chem. Abstr., 2001, v. 135, 332724u. 105. Sharkey W.N. Fluorine Chem. Rev. New York, 1968, v. 2, p. 1-54. 106. Linkind M.B. Ref. Diss. Kand. Khim. Nauk. M., 1987. 42P. 107. Linkind M.B., Volchkov N.V., Shipilov A.I., Zabolotzkii V.F. Tez. Dokl. VI Vsesoyuzn. Conference in Khimia fluoroorganic compounds. Novosibirsk. 1990, p. 26. |
Fluorine Notes, 2006, 46, 1-2
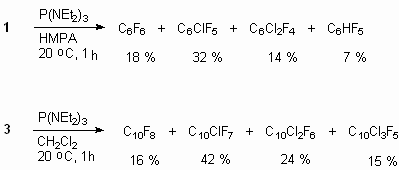
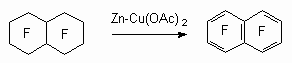
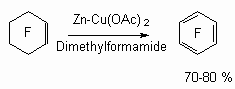
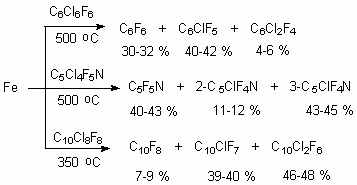
 5C5H5)2TiF2]
or [(
5C5H5)2TiF2]
or [(