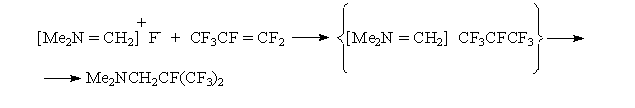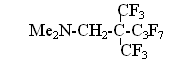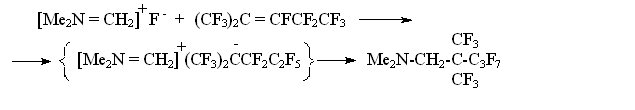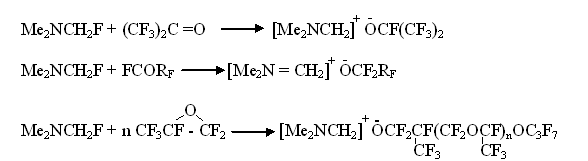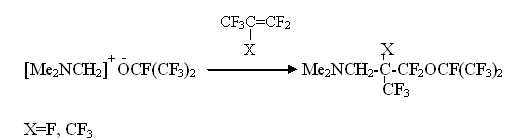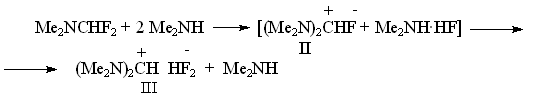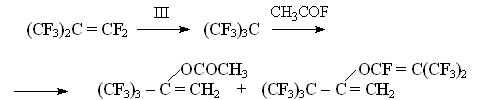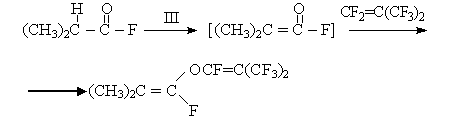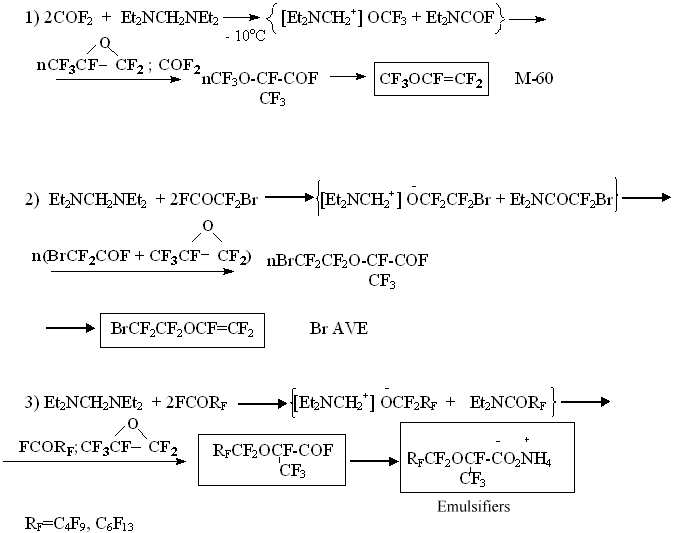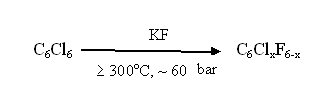Fluorine Notes, 2006, 46, 3-4
|
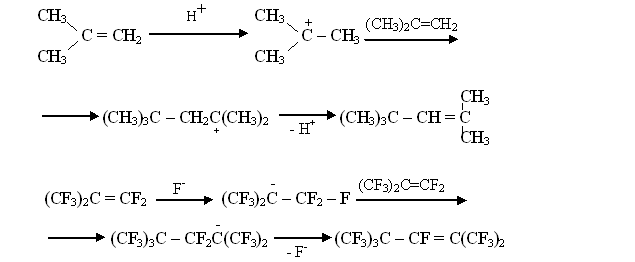
α- FLUOROALKYLAMINES AS NEW SOURCES FOR UNHYDRATED FLUORIDE- ION S.M.Igoumnov Scientific and Production Association "P&M Invest" This article opens the subject of synthesis and application of fluoroalkylamines for the obtaining reactions of fluoroaliphatic and fluoroaromatic compounds. I.L. Knuniantz was a staunch supporter to the fact, that anion fluorine would have the same enormous significance for the chemistry of fluororganic compounds as proton for the chemistry of organic compounds. The analogy of fluorolefines' transformations in the presence of Bronsted's acids and of perfluorolefines' transformations in the presence of fluorine anion [1] was cited as a proof. In an enormous number of works by colleagues and followers of I.L. Knuniantz the practical importance of processes involving fluorine anion [2-5] was displayed and their theoretical aspects were studied. For the present moment, these processes are among the main tendencies of preparative and commercial synthesis of fluororganic compounds. However, the picking up of sources of fluorine anion was limited by cesium fluorides, rubidium and potassium fluorides. From the economical point of view, such choice for large-scale synthesis is limited only by potassium fluoride. In its turn, the fluorides' low solubility in the mediums suitable to carry out syntheses with anion fluoride had limited the opportunities of the method. In a view of that, I.L. Knuniantz had set a goal to find new sources of anion fluoride allowing extending the field of application for such processes. In 1979 the researches had begun. As a result of studies it was found, that in polar solvent α-fluorotrimethylamine (covalent compound at ordinary conditions is liquid, which boiling point equals 47oC, and it is easy to dissolve it in polar and non-polar solvents) easily adds according to perfluorolefines' double bond forming stable products [6]. Besides that, it turned our that in NMR- spectra of α-fluorotrimethylamine's solutions in polar solvents H-F interaction doesn't take place. Taking these facts as a foundation we made a presumption, that dissociation of α-fluorotrimethylamine's molecule into ions occurred in polar solvents [7]: This assumption allows to realize the easiness of addition of α -fluorotrimethylamine to fluorolefines. It's obvious, that here we encounter an anionic reaction scheme: The suggested scheme is proved by forming of by-product that is (2-trifluoromethylperfluoroamyl-2)trimethylamine Perfluorinated radical is a derivative of perfluoropropylene dimer, formed out of perfluoropropylene in the presence of anion fluoride [8]:
At the next stage α-fluorotrimethylamine adds perfluoropropylene dimer: Thus, it became clear, that α-fluorotrimethylamine is a source for anion fluoride for highly electrophilic fluorolefines. After that the facts, that perfluorinated O-anions at interaction of fluorotrimethylamine and perfluorinated carbonyl compounds were formed had been taken with no surprise [9-12].
The formed ionic compounds add according to double bond of fluorolefines forming covalent compounds
The easy dissociation of stable C-F bond in α-fluorotrimethylamine must be determined by the presence of nitrogen heminal atom:
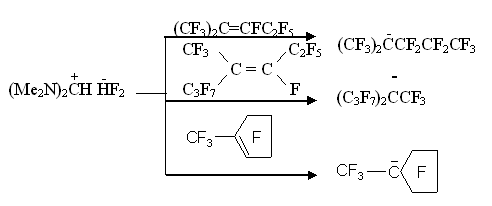
It was natural that an interest for studying a number of such compounds and for comparing their characteristics as sources for anion fluoride arose. N,N,N',N'- tetramethylformamidinium fluoride (bisdimethylaminofluoromethane) (II) was synthesized for this purpose, which is such a strong base, that eliminates HF from dimethylamine fluorohydrate forming N,N,N',N'-tetramethylformamidinium bifluoride (III) [11] Obtained formamidinium bifluoride proved to be such a powerful source for fluorine anion that having used it we for the first time managed to synthesize a number of perfluoroalkylcarbanions and to study their NMR-spectra:
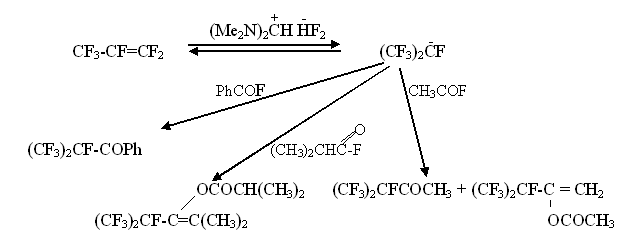
It was stated, that perfluoroalkylcarbanions generated this way enter typical for carbanions reactions:
We also managed to generate and fully acylate perfluorotertbutylanion
Fluorine-anion produced out of formamidinium bifluoride proved to be a foundation strong enough to enable enolazation of isobutyryl fluoride:
A studying of tris-dimethylaminofluoromethane (IV) was a next logical step to take. It was obtained by the reaction of hexamethylguanidinium pivaloate and dimethylaminotrifluorosulphurane .
It was found, that (IV) as (III) exists only in ionic form of hexamethylguanidinium fluoride. As it should have been expected (IV) is a great source for anion fluoride for generating perfluoroalkylcarbanions out of perfluorolefines. It practically irreversibly adds to perfluoroisobutylene forming stable salt:
It for the first time allowed acylation of perfluorotertbutylanion forming phenylperfluorotertbutylcetone [13]:
It is known, that the last compound mentioned easily decomposes in the presence of catalytic quantities of fluorine anion [14]:
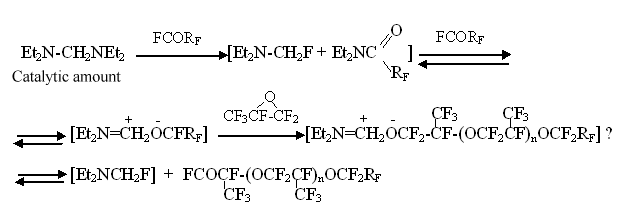
The formation of phenylperfluorotertbutylcetone in the example listed above indicates the absence of F- in reaction mixture and the irreversibility of (IV) addition to perfluoroisobutylene. Thus, in laboratory of I.L. Knuniantz under his direct supervision a theoretical foundation of obtaining and application of fundamentally new, well dissoluble in organic solvents, high- performance source of fluorine anion was laid. The knowledge obtained before found their practical realization. Thus, the ability of dialkylaminofluoromethanes to serve as a source of fluorine anion in reactions with perfluorocarbonyl compounds was put into practice at a pilot plant of Russian Scientific Center of Applied Chemistry Perm Branch for obtaining technologies of perfluorovinyl ethers and surface active materials [15]:
N,N,N',N' - tetraethylmethylenediamine is used in catalytic quantities for these processes. The application of the catalysis method worked out allowed to put into practice many processes with perfluorinated O-anions participating in at temperature ranging from - 10oC to room temperature and at atmospheric pressure. Based on these foundations the following production processes are being carried out at Perm Branch of RSC of Applied Chemistry:
At the same time using of other sources of fluorine anion for many of analogous processes requires elevated temperatures (90- 150oC) and pressure (5 - 200 bar) [16]. The unique properties of new sources of fluorine anion found their application also for the production of fluoroaromatic compounds. Thus, the fluorinating process of hexafluorobenzene using potassium fluoride, worked out by G.G. Jacobson [17] requires high temperatures and therefore high values of pressure:
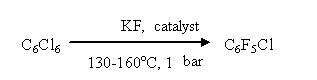
It limits the volume of reactors (autoclaves) and makes it impossible to organize a large-scale production. Using hexaethylguanidium fluoride as a catalyst for this process allows decreasing of temperature down to 130 - 150oC and carrying out the process with no pressure at conditions of uninterrupted isolation of target products [18]
The technology worked out allowed to set up commercial production of fluoroaromatic compounds at Perm Branch of RSC " Applied Chemistry". Thus, theoretical works regarding search for new sources of fluorine anion carried out at I.L. Knuniantz laboratory under his direct supervision found and there is no doubt, that later will be finding their wide practical application. References 2. Dyatkin B.L., Mochalina E.P., Knunyants I.L., Izv. AN SSSR., Ser. khim., 1967. № 2, s. 469. 3. Sterlin S.P., Zhuravleva L.G., Dyatkin B.L., Knunyants I.L., Izv. AN SSSR., Ser. khim., 1971, № 11, s. 2517-2521. 4. Rohlin E.M.. Volkonskij A.Yu.. Mysov E.N, Izv. AN SSSR., Ser. khim., 1978, № 6, s. 1359-1363. 5. Zeifman Yu.V., Lantseva L.T., Knunyants I.L. Izv. AN SSSR., Ser. khim., 1978, № 11, s. 2640-2643. 6. Knunyants I.L., Delyagina N.I., Igumnov S.M., Izv. AN SSSR. Ser. khim., 1981, № 4, s. 857-859 7. Igumnov S.M., Diss...kand. khim. nauk. Moscow, INEOS AN SSSR, 1983. 8. Kopaevich Yu.L., Belen'kij G.G., Mysov E.I., German L.S., Knunyants I.L., ZhVKhO im. D.I. Mendeleeva, 1972, t. XVII, № 2, s. 236. 9. Knunyants I.L., Delyagina N.I., Igumnov S.M., Izv. AN SSSR., Ser. khim., 1981, № 4, s. 860-863. 10. Delyagina N.I., Igumnov S.M., Snegirev V.F., Knunyants I.L., Izv. AN SSSR., Ser. khim., 1981, № 10, s. 2238-2243. 11. Igumnov S.M., Delyagina H.I., Knunyants I.L., Izv. AN SSSR., Ser. him., 1981, № 10, s. 2339-2342. 12. Igumnov S.M., Delyagina N.I., Zejfman Yu.V., Knunyants I.L., Izv. AN SSSR., Ser. khim., 1984, № 4, s. 827-832. 13. Igumnov S.M., Delyagina N.I., Knunyants I.L., Izv. AN SSSR., Ser. khim., 1986, № 6, s. 1315 1317 14. Knunyants I.L., Zejfman Yu.V., Lantseva L. G., Dokl. AN SSSR., 1980, t. 254. № 1, s. 117-120. 15. Igumnov S.M., Lekontseva G.I., Shipigusev A,A., Muhametshin V.F., Zh. prikl. khimiya, 2005, t. 78, № 3, s. 438-440. 16. Sintezy ftororganicheskih soedinenij., pod red. I.L. Knunyantsa i G.G. Yakobsona. M; Khimiya, 1973, 312 s. 17. Vorozhtsov N.N.-jr., Platonov V.E., Yakobson G.G., Izv. AN SSSR., 1963, № 8, s. 1524. 18. Igumnov S.M., Zabolotskih A.V., Patent RU 2164508, Publ. 27.03.01. |
Fluorine Notes, 2006, 46, 3-4