Fluorine Notes, 2006, 45, 1-2
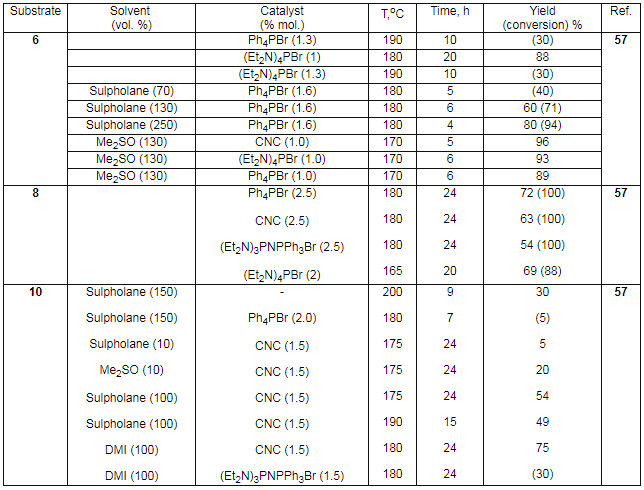
PRESENT-DAY CONDITION OF FLUOROAROMATIC COMPOUNDS PRODUCTION TECHNOLOGYG.G. Furin a *, L.E. Deev b a* Novosibirsk Institute of Organic Chemistry , Russian Academy of Science 630090, Novosibirsk, Ac. Lavrentiev av. 9, e-mail : furin@nioch.nsc.ru b Perm State Agricultural Academy, 614990, Perm, e-mail : deev@permonline.ru Here we describe effectiveness of interphase transfer catalysts use to obtain polyfluoroaromatic compounds by potassium fluoride influence on polychlorbenzenes. Such catalysts as hexaethylguanidine chloride, tetra-(diethylamino)- phosphonium bromide are involved into stabilization of intermediate s-complex. Catalytic participation of polyethers (tetraethyleneglycole dimethyl ether, 18-crown-6) in fluorodechlorinating process doesn't go beyond increasing of "active" fluoride-ion concentration. Here we consider the opportunities of mechanic and chemical technology application to synthesize fluoroaromatic compounds by substituting chlorine for fluorine in the solid phase of chloroaromatic compounds and fluorides of alkali, alkali-landed metals and composite mixtures based on them. We also discuss the question regarding synthesizing fluoroaromatic compounds out of commercial chladones (freons) and polyfluorolefines. Contents Introduction 1. Hexafluorobenzene synthesis by potassium fluoride influencing hexachlorobenzene in the presence of catalysts. 2. Mechano-chemical obtaining method of hexafluorobenzene. 3. The using of polyhaloidbenzenes fluorination and fluorination products' dehalogenation processes as obtaining method of hexafluorobenzene and other aromatic compounds. 4. Synthesis of fluoroaromatic compounds out of commercial chladones and polyfluorolefines. Conclusion References (Continuation). The presence of two nitro-groups in benzene ring forwards chlorine exchange for fluorine under the influence of KF, though the process passes at high temperature, while the use of Ph4P+.Cl- salt as a catalysts allows lowering the temperature of process [44]. Such salts based on aminophosphazene as 1,1,1,3,3,3-hexapyrrolidino-diphosphazenium chloride chloride and 1,1,1,3,3,3-hexapiperidino- diphosphazenium chloride are effective catalysts for chlorine exchange for fluorine under the influence of alkali metals' fluorides or ammonium fluoride. Thus, the reaction of 2,5-dichloro-2,4,6-trifluoropyridine and KF in the sulpholane -chlorobenzene solvents' system at 215 oC produces 3-chloro-2,4,5,6-tetrafluoropyridine with the yield of 75% and pentafluoropyridine with the yield of 24% in the presence of these salts [44]. 2,6-Difluorobenzaldehyde was obtained out of 2-chloro-6-fluorobenzaldehyde by KF influencing in chlorobenzene at 190 oC , it's yield was about 88.7 - 88.5 % [44]. The carrying out of chlorine for fluorine exchange process in para-chloronitrobenzene proved to be effective when carrying the process out in bipolar aprotic solvents in the presence of Ph4PBr and 4 (Table 7).
Table 7. Fluorinating the Benzene Chlorine Derivatives by Potassium Fluoride in the Presence of Catalysts The presence of two chlorine atoms in benzene ring produces, as a rule, difluoroderivatives, for example in case of 2,6-dichlorobenzaldehyde (table 7) they obtain 2,6-difluorobenzaldehyde, which is an important product for herbicide production [57].
The same result is obtained also for 2,4- dichlorobenzoic acid dichloranhydride (table 6) [57].
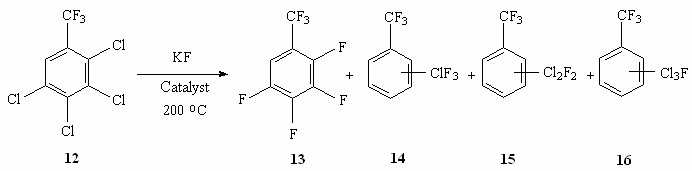
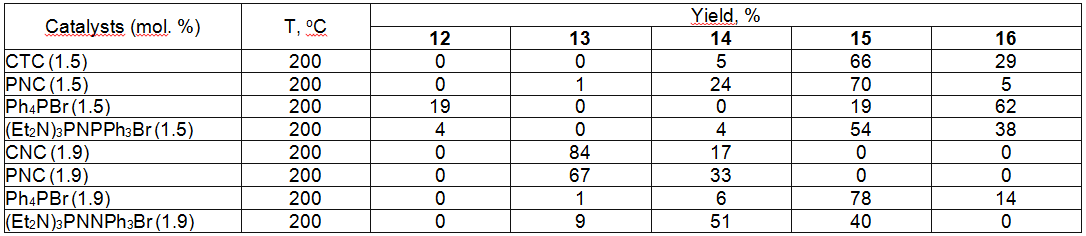
We can observe a more complicated picture at fluorinating of tetrachlorobenzotrifluoride 12. In this case depending on the character of catalysts used we see a different degree of fluorination (table 8) [57].
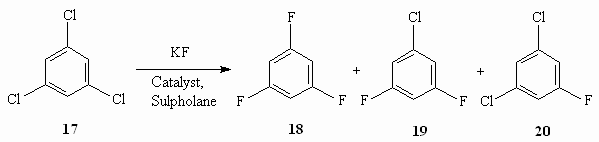
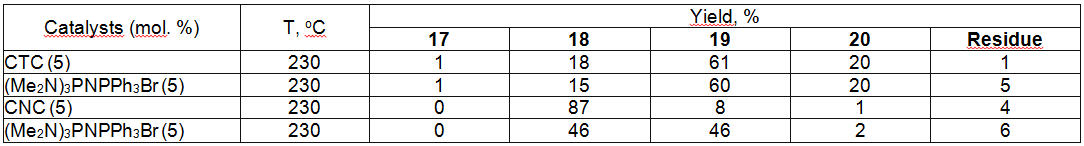
Table 8. Synthesis of Tetrafluorobenzofluoride out of Tetrachlorobenzotrifluoride [57] Effective catalysts have been found for production of 1,3,5-trifluorobenzene by influencing of potassium fluoride on 1,3,5-trichlorobenzene (table 9) [57].
Table 9. Obtaining of 1,3,5-trifluorobenzene by Influencing 1,3,5-trichlorine-benzene Using Potassium Fluoride in the Presence of Catalysts [57] Thus, 2,3,4-trifluoro-5-chloro-1-trifluoromethylbenzene is obtained with the yield of 85% at heating of 2,3,5-trichloro-1-trifluoromethylbenzene in sulpholane in the autoclave at 210 oC and pressure of 2.2 bar in the presence of Et4PBr [58]. The effectiveness of process is increasing when using fine-loosened anhydrous potassium fluoride and tetrakis(diethylamido)-phosphonium bromide (R2N)4P+ . Hal- [59,60]. Taking into account a commercial worth of hexafluorobenzene the majority of information regarding its obtaining has been published as patents, which sometimes hardly reveal the essence of offered technologies. Nevertheless the effectiveness of catalysts use to intensify the known hexafluorobenzene obtaining method is determined. In this case, we manage to soften the tough conditions of synthesis or to refuse the using of solvents. It is stated, that quaternary ammonium salts, guanidine derivatives, quaternary phosphonium salts, krypton derivatives, crown-ethers and polyesters [30,44-48,61,62] evince their most effectiveness as catalysts for intrerphase transfer. Thus, the authors of work [30] showed, that potassium fluoride applied over calcium or barium fluoride reveals its high activity in the reaction of hexafluorobenzene fluorination. At that, the simultaneous using of such potassium fluoride and quaternaryphosphonium salt (for example, tetrakis(diethylamido)-phosphonium bromide) essentially increases the efficiency of chlorine for fluorine exchange process (table 2). As we can see from the table 2, under conditions (230 oC, 42.5 h) the using of potassium fluoride dried by dispersion produces the summary yield of fluorination products of only 6%, while when using potassium fluoride, applied over calcium fluoride under analogous conditions produces the mixture of subsequent substitution of chlorine for fluorine products of high yield, though the main product is tetrachlorodifluorobenzene (46 %). The substitution of chlorine for fluorine in hexachlorobenzene goes notably easier under the influence of KF when using tetrakis(diethylamido)-phosphonium bromide as a catalyst for inter-phase transfer: at 230 oC in 42.5 h the reaction products contain 13.5% of chloropentafluorobenzene, 37% of dichlorotetrafluorobenzene and 45% of dichlorotetrafluorobenzene, which is the main product. Hexachlorobenzene fluorination process is going much faster when using potassium fluoride, applied on CaF2(Table 2 ). The obtained results can be a consequence of fluorination both on a solid surface and at organic phase and factors increasing the absorption of reagents on a solid surface (pressure, substrates nature) will lead to raising the rate of exchange. N,N',N''-Hexasubstituted guanidinium chlorides proved as effective ones at obtaining hexafluorobenzene [59,63]. Thus, hexafluorobenzene was obtained out of hexachlorobenzene and KF with the yield of 90% at 160-170 oC in the presence of this catalyst. We used ammonium salts like [(RR'N)2CNR2R3]+ . X- (where R-R3 = alkyl C1-7, cycloalkyl C5-8) or hexaethylguanidinium chloride [64] as catalysts. Carrying out reaction at 160-170 oC produces the mixtures, containing 91% of hexafluorobenzene, and 9% of partly fluorinated fluorochlorobenzenes. The overall yield of hexafluorobenzene is 88.4%. The quaternary phosphonium salts are more effective, what caused their wider use and research of exchange processes involving them. Thus, pentafluoro-containing benzene derivatives like C6FnX6-n ( X = F, Cl, CF3, CN; n = 1-5) are obtained by heating of corresponding haloid containing aromatic compounds with alkali metals fluorides in the liquid phase in the presence of tetrakis(diethylamido)phosphonium bromide in the medium of products of starting substrate partial fluorination with simultaneous selection of target products at 150-200 oC [65]. In this case the technological process is becoming greatly simpler and the yield of polyfluoroarimatic compounds increases. When carrying out a process of hexafluorobenzene fluorination using potassium fluoride in benzonitrile (catalyst amount is 12 g per 115 g of hexachlorobenzene) at 200 oC in 5.5 h we obtained a mixture, containing 24.4% of hexafluorobenzene, 39.9% of pentafluorochlorobenzene, 21% of tetrafluorodichlorobenzenes' mixture and 8% of trifluorotrichlorobenzenes [60]. The effectiveness of this catalysts was proven by authors of works [55,66] using example of hexachlorobenzene and partly fluorinated chlorobenzenes. We should note, that the presence of several chlorine atoms in benzene ring is not an obstacle for exchange reaction taking place [55].
Catalysts can take part in acceleration reaction of fluorodechlorination of chloroaromatic substrates at different process stages. At first, they can increase KF concentration in liquid phase, secondly, lowering the energy of reaction activation because of inter-phase This method proved to be so effective, that American company "Albemarle" not only has patented the technology of synthesis of fluorinated aromatic compounds, allowing to raise the yield of final products at lower temperatures and at more moderated figures of pressure compare to usual method and to diminish the reaction period, but it also has built two plants producing several tons of hexafluorobenzene per year [67,68]. Different benzene derivatives are obtained based on it and first of all one of them is pentafluorobromobenzene.. When using tetraethylene glycol dimethyl ether and 18-crown-6 the catalysis effectiveness depends on substrate origin - the isomeric composition of trifluortrichlorobenzenes, forming at using these catalysts and without their using in sulpholane, at the same degrees of conversion is approximately the same [69] and in turn is close to isomeric composition at fluorination of trifluorotrichlorobenzenes using potassium fluoride at 350 oC. The authors consider, that speeding up of chlorine substitution for fluorine mainly occurs because of potassium fluoride nucleophilic reactivity rising. Fluorodechlorination of tetrafluorodichlorobenzene by KF goes slowly (only traces of pentafluorochlorobenzene can be seen in 6 hours) in the presence of catalytic amounts of diglyme, tetraglyme or 18-crown-6 ester. [59]. The combination of hexaethylguanidinium chloride with polyesters results in noticeable growth of activity of catalytic system (conversion of terafluorodichlorobenzene doubles). We should notice, that such a growth of activity was mentioned by the authors of work [30] when adding polyester to KF, applied on inert supporter (CaF2, BaF2) at fluorinating of hexafluorobenzene. When using polyesters as catalysts the catalytic effect is explained from the point of view of increasing of "active" ion -fluoride current concentration, which leads to speeding up of fluorodechlorination [59]. When using other catalysts the catalytic effect has some other origin and is connected not only with increasing of concentration of "active" ion- fluoride, but also with great participation of these catalysts in stabilization of anionic
Fig.1. The depending of conversion degree on reaction period (mole proportion C6Cl2F4 : KF : catalyst, 1:1:0.05) [70] 1 - hexaethylguanidinium chloride 2 - tetra(diethylamido)phosphonium bromide 3 - 18-crown-6 4 - tetraethylene glycol dimethyl ether 5 - sulpholane (mass ratio C6Cl3F3 : sulpholane , 1:1). At the same time in case of catalysts of tetra(diethylamido)phosphonium bromide and hexaethylguanidiniumchloride the difference of isomeric compositions compare to non-catalytic variant becomes noticeable as soon as conversion reaches 8.5%, and mainly developing at conversion of 20%. The appearance of catalytic effect of these catalysts depends to a great extent on the origin of substrate. It can be connected with the scheme differences, taking place in every case of the processes [64]. According to up-to-date conceptions, the process of chlorine-aromatic compounds fluorodechlorination using potassium fluoride goes both on the surface of solid phase and in There are estimations, that poly-fluorochlorobenzenes' fluorodechlorination goes according to the addition - eliminating scheme with intermediate forming of anionic The information cited here allow us to establish the effect of applying catalysts at obtaining fluoroaromatic compounds by alkali metals fluorides influencing chlorine containing aromatic compounds. One of the ways to improve effectiveness of aromatic compounds fluorodechlorination process is its accompanying by physical impact. Thus, using an example of reaction of nucleophilic chlorine replacement for fluorine we demonstrated a fundamental opportunity of carrying out a solid phase synthesis of fluoroaromatic compounds under conditions of mechanical and chemical activation. Besides that, liquid phase fluorination processes can also be intensified by using fluorinating agent activated beforehand. An important role here belongs to the preliminary preparation of potassium fluoride, which microdispersion and effective surface are particularly essential [70,73-75]. It was demonstrated, that when carrying out a reaction of hexachlorobenzene, octachloronaphtalene, pentachloropyridine and potassium fluoride or other fluorides of alkali and alkaline-earth metals and also composites of a mixture containing (KF-CaF2, K2CO3-CaF2 [76]) made on their base in planet-friction activator (planet mill APF-1M), which forwards mechanical and chemical activation of potassium fluoride (acceleration is 40 g, activativation period 20 min) at 350-500 oC we can obtain a mixture of chlorofluorobenzenes, octafluoronaphtalene and pentafluoropyridine respectively. KF mechanical activation leads in acceleration of fluorination process and allows to increase yields of poly-fluorinated benzenes or to shorten the period of reaction [70, 73]. Octafluoronaphtalene can be fluorinated most easily, and hexachlorobenzene is a most difficult to be fluorinated. Thus, in 20 minutes activation period the rate of transformation of octachloronaphtalene into fluorine derivatives amounted to 75%, while in the same period the rate of hexachlorobenzene amounted only to 22%. As the period of treatment was being increased we observed a grow of conversion and substitution rate. The application of other metals fluorides in this reaction allowed to make a conclusion, that activity of fluorinating agent was growing from lithium fluoride to cesium fluoride and from calcium fluoride to barium fluoride. However in all the cases we observe a complicated composition of partly substituted products. The mixture was accumulated and rectified in an ordinary way to isolate individual products. This approach allows decreasing the process period, the excess of applied potassium fluoride and amount of waste products. It is found, that in potassium fluoride-calcium fluoride systems and also potassium carbonate - calcium fluoride the formation of mixed fluoride of KCaF3, occurs, which is a new fluorinating agent of a high activity. Thus, using a composite mixture containing KF + CaF2the reaction of pentachloropyridine fluorination actually goes only in 30 minutes, while when using KF the products' yield is 35,8% in 120 min, and CaF2 - 14.8%. Compare to potassium fluoride the mixed fluoride shows higher fluorinating ability not only during mechanical and chemical, but particularly during liquid phase process of fluorination. Using of fluorinating reagent (KF and KCaF3) allowed to obtain 3-chlorine-4-fluoronitrobenzene out of 3,4-dichloronitrobenzene at 150 oC in the sulfolane medium [73]. |
Fluorine Notes, 2006, 45, 1-2
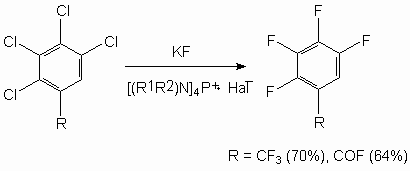
 -complex participation in stabilization, they can also lead to increasing of catalytic system activity [59]. Adding to such catalytic system promoters, representing compounds of heterocyclic cycle and aromatic row, ethers, substituted amides of organic acids etc, promotes the rate of chlorine exchange for fluorine and increasing the yield of final products [62]. Under these conditions the compounds with the common formula C6F4XY (where X = F, Cl, CF3, CCl3, CN, COR; Y = F, H, Cl, CF3, CCl3, CN, COR) were obtained with their high yields [62]. The proportion of quaternary tetra-amidophosphonium salt is in the range between 100 : 1 and 5-20 : 1, and proportion of alkali metal fluoride and quaternary tetra-amidophosphonium salt is in the limits ranging from 1000 : 1 to 10 : 1.
-complex participation in stabilization, they can also lead to increasing of catalytic system activity [59]. Adding to such catalytic system promoters, representing compounds of heterocyclic cycle and aromatic row, ethers, substituted amides of organic acids etc, promotes the rate of chlorine exchange for fluorine and increasing the yield of final products [62]. Under these conditions the compounds with the common formula C6F4XY (where X = F, Cl, CF3, CCl3, CN, COR; Y = F, H, Cl, CF3, CCl3, CN, COR) were obtained with their high yields [62]. The proportion of quaternary tetra-amidophosphonium salt is in the range between 100 : 1 and 5-20 : 1, and proportion of alkali metal fluoride and quaternary tetra-amidophosphonium salt is in the limits ranging from 1000 : 1 to 10 : 1. -complexes, that results in decreasing of activation fluorodechlorination reaction energy probably because of more effective stabilization of inter-phase
-complexes, that results in decreasing of activation fluorodechlorination reaction energy probably because of more effective stabilization of inter-phase -complex [59].
-complex [59].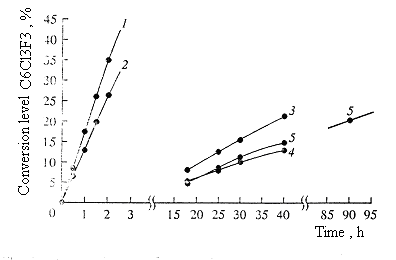
 -phase, directly bordering to potassium fluoride [16]. In both cases, the catalytic effect is connected with increase of reactivity of potassium fluoride. In first case, as they think, it occurs because of more effective coordination of substrate on the surface of potassium fluoride, and in the second one the reason of it is forming of incoherent ionic pares between catalyst and ion-fluoride [16].
-phase, directly bordering to potassium fluoride [16]. In both cases, the catalytic effect is connected with increase of reactivity of potassium fluoride. In first case, as they think, it occurs because of more effective coordination of substrate on the surface of potassium fluoride, and in the second one the reason of it is forming of incoherent ionic pares between catalyst and ion-fluoride [16].