Fluorine Notes, 2006, 45, 5-6
REACTIONS OF 5,6-SUBSTITUTED URACYLS WITH ELEMENTAL FLUORINE AND PROPERTIES OF THUS PREPARED SUBSTANCES
S.G.Semenov, B.N. Maximov
FSUE Russian Scientific Center "Applied Chemistry",
192198, St.Petersburg, 14, Dobrolubov ave;
E-mail: boris_maximov@mail.ru
The methods of thin-layer chromatography, liquid high-resolution chromatography, IR-, UV-, and NMR 1H and 19F spectroscopy were used to study the composition of the products resulting from the interaction between uracyl or 5,6-substituted uracyls (substituents were CH3, F, Cl, Br, or NO2) or 6-aza-uracyl and elemental fluorine in anhydrous hydrogen fluoride at variable reaction conditions and reagent concentrations.
It is shown that 5-fluorouracyl (uracyls) are not formed in the case of "6"-position of the substituent, when fluorine molecule is added to the double bond in positions 5, 6:
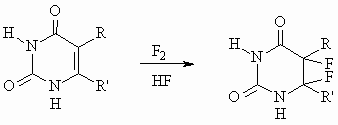
The resulting 5,6-difluoroderivatives of substituted uracyls are quite stable (with the exception of 6-azauracyl); their hydrolysis, alcoholysis and acydolysis results in 5-fluoro-6-substituted-5,6-dihydrouracyls, e.g.:
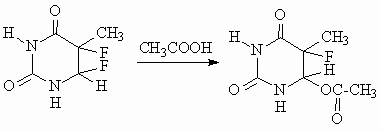
The structures of those products are confirmed by their counter-synthesis through the treatment of substituted uracyls with fluorine in appropriate solvents (water, alcohols, or carboxylic acids).
Those substances pyrolysis results in the formation of 5-fluorouracyl isomers, i.e.:
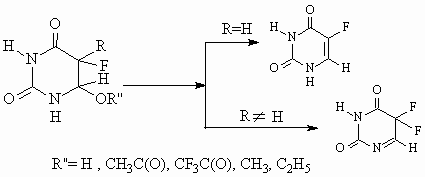
The fine structure of those substances is studied with the help of physico-chemical methods, and it is shown that some products of fluorine addition contain the mixture of cis- and trans-isomers.
Fluorine Notes, 2006, 45, 5-6
