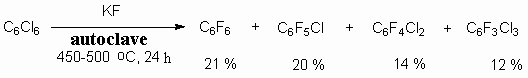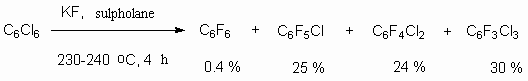Fluorine Notes, 2006, 44, 1-2
PRESENT-DAY CONDITION OF FLUOROAROMATIC COMPOUNDS PRODUCTION TECHNOLOGYG.G. Furin a *, L.E. Deev b a* Novosibirsk Institute of Organic Chemistry , Russian Academy of Science 630090, Novosibirsk, Ac. Lavrentiev av. 9, e-mail : furin@nioch.nsc.ru b Perm State Agricultural Academy, 614990, Perm, e-mail : deev@permonline.ru Here we describe effectiveness of interphase transfer catalysts use to obtain polyfluoroaromatic compounds by potassium fluoride influence on polychlorbenzenes. Such catalysts as hexaethylguanidine chloride, tetra-(diethylamino)- phosphonium bromide are involved into stabilization of intermediate s-complex. Catalytic participation of polyethers (tetraethyleneglycole dimethyl ether, 18-crown-6) in fluorodechlorinating process doesn't go beyond increasing of "active" fluoride-ion concentration. Here we consider the opportunities of mechanic and chemical technology application to synthesize fluoroaromatic compounds by substituting chlorine for fluorine in the solid phase of chloroaromatic compounds and fluorides of alkali, alkali-landed metals and composite mixtures based on them. We also discuss the question regarding synthesizing fluoroaromatic compounds out of commercial chladones (freons) and polyfluorolefines. Contents Introduction 1. Hexafluorobenzene synthesis by potassium fluoride influencing hexachlorobenzene in the presence of catalysts. 2. Mechano-chemical obtaining method of hexafluorobenzene. 3. The using of polyhaloidbenzenes fluorination and fluorination products' dehalogenation processes as obtaining method of hexafluorobenzene and other aromatic compounds. 4. Synthesis of fluoroaromatic compounds out of commercial chladones and polyfluorolefines. Conclusion References The success of contemporary scientific and technical revolution using of its achievements in practice are inseparably tied together and are to a great extent caused by the progress reached in the fields of new chemical compounds synthesis and commercial production methods working out. All that can be explained by the fact that the variety of structural, physical, and chemical characteristics of organic compounds and materials based on them is much larger than of traditional non-organic products and metals. Fluororganic compounds attract attention of many organic chemists and specialists of other fields of science because of their unique properties, that determined the perspectives of their use to solve a number of chemistry principal theoretical issues and industrial and medical goals. Being a new filed of organic chemistry, created solely by human's mind and hands, the chemistry of fluororganic compounds even by the present time has played an important role for the progress of several branches of industry, it also has produced new, unknown in the nature materials and compounds, possessing a number of specific properties. Mainly this refers to polyfluorinated organic molecules. Accumulating of fluorine atoms in carbon skeleton of molecule leads to formation of new properties. At that, the introduction of other elements atoms does not largely allow to reach them. It provides an opportunity to create new fluorine materials. Poly-fluorinated compounds of aliphatic row found a most wide application, while poly-fluoroaromatic derivatives are being used to a smaller extent in practice. It was considered, that polyfluoroaromatic derivatives didn't have any practical value and were out of interest. However, time proved this statement incorrect. Thus, polyfluorobenzoic acids are widely used to create synthetic antibiotics (fluoroquinolones), pentafluorobrombenzene is used to synthesize tris(pentafluorophenyl)boron used as co-catalyst to create highly effective catalysts for polymerization of ethylene and propylene and to obtain stable xenon compounds of the C-Xe bond. Octafluorotoluene is used as semi-product for synthesis of fluororubbers, used for aviation and for synthesis of oxygen carriers ("blue blood") etc. This mainly is related to our relatively narrow knowledge of them and lack of perfection in their obtaining methods. Only in recent years, the field of their practical application and of fluorine materials based on them began to develop intensively. Poly-fluoroaromatic compounds are known for a rather long period of time as a class of aromatic compounds. In a contemporary development of polyfluoroaromatic compounds chemistry we can separate out two main tendencies. First is related to search for synthesis methods of poly-fluoroaromatic derivatives and to studying of their reactivity. Here we have to mark the working out of a simple and most convenient obtaining method of hexafluorobenzene, which was carried out in the USSR by N.N. Vorozhtsov and G.G. Yakobson. The method is an exchange of chlorine atoms inside polychloroarenes for fluorine atoms under the influence of potassium fluoride [1-3]. It had become a base for expanding a complex of researching regarding studying of poly-fluoroaromatic compounds chemical characteristics and it had opened practically unlimited opportunities to create new materials based on them [1]. Second tendency is related to increased interest for hetero-organic compounds posessing pentafluorophenyl ring. If an obtaining technology of polyfluorinated benzene derivatives has nevertheless been created, we can't say so about low-fluorinated derivatives. Due to the limited number of obtaining methods of partly fluorinated benzene derivatives the problem of working out approaches for synthesis of low-fluorinated benzene derivatives is increasing. Such approach is justified by the fact, that many drugs, biologically active compounds, dies etc contain one or two fluorine atoms. One of approaches to obtain them is use of poly-fluoraromatic compounds as raw materials. That's why it is not surprising, that many works are devoted to their transformation into low fluorinated. Low- fluorinated benzenes are obtained using different methods, among which we can notice fluorination either by elemental fluorine in trifluoromethansulpho-acid [4], or in sulphuric acid [5], or by fluorine carriers according to Schiemann reaction in anhydrous hydrogen fluoride [6]. The analysis of these methods has been carried out rather in details in monography [6] and here we do not review it. A simplified and more economical method of fluorobenzene manufacturing has been worked out. It is based on fluorination of benzene using anhydrous hydrogen fluoride and oxygen in the presence of CuF2 catalyst [7,8]. The process is carried out in tube nickel reactor in two stages. At first stage the copper oxide is being fluorinated under the influence of anhydrous hydrogen fluoride till CuF2 at 400 oC. At the second stage the flow of benzene (2 cm3/min) and nitrogen (20 cm3/min) are being run through catalyst at 450-550 oC to produce fluorobenzene and to reduce CuF2 up to metal copper. The selectivity of formation of fluorobenzene exceeds 95 %, the conversion of benzene is 30% at 550 oC. The conversion of benzene is decreasing in time due to reducing CuF2 to metal, which covers the particles of catalyst. The present method can also be used to synthesize other benzene low fluorine derivatives, for example fluorotoluenes, difluorobenzenes [9], pyridine [8]. Key works regarding working out obtaining method of totally fluorinated hexafluorobenzene [1,2] and octafluoronaphtalene [3] by anhydrous potassium fluoride influencing hexafluorobenzene and octafluoronaphtalene in circulating autoclave at 450-500 oC were carried out under the direction of of N.N. VorozhtsovandG.G. Yakobson in Russia.
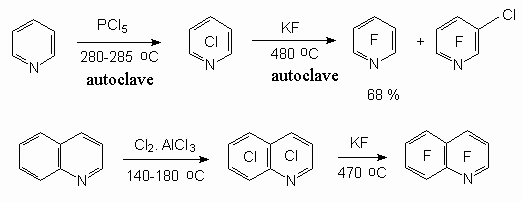
Method was used for heterocyclic compounds, for example pyridine [10-12] and quinoline derivatives [13].
The problem of poly-fluoroaromatic compounds is described in books [14-17] and reviews [18,19]. The reaction can be carried out also in a liquid phase way in solvents like dimethylformamide and sulpholane at air pressure. Thus, carrying out a process in sulpholane at 230-240 oC produced the same mixture of products as while using a "dry" method. However, hexafluorobenzene was obtained with the yield equal only to 0.4%, while the yield of octafluoronaphtaline was about 52% [21]. The disadvantages of reaction's carrying out in solvent are its high cost, and also the need to either regenerate it or to eliminate it after carrying out the synthesis.
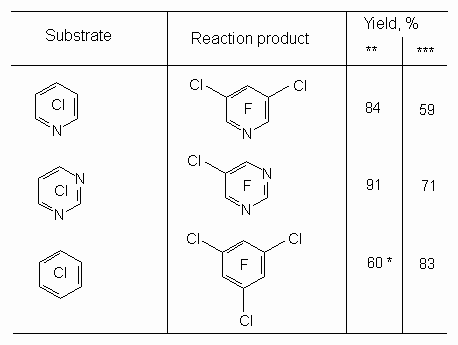
The picking out of solvent system allows to increase greatly the yield of perfluorinated derivatives of aromatic compound. The information on interaction between potassium fluoride and chlorine derivatives in the presence of crown-ether or sulpholane in perfluoroperhydrophenanthrene (b.p. is 215 oC) (table 1) can be an example. [22,23]. Table 1.Fluorination of organochlorine derivatives by KF in perfluoroperhydrophenanthrene solution ( 190 oC, 15 h) [21]
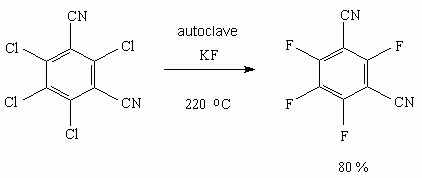
* 217 h, ** 18-crown-6, *** tetrahydrothiophene 1,1-dioxide (25 %). Japanese researchers actively used sulpholane as solvent and benzonitriles as starting substrates, which had undergone chlorination in advance. Thus, while dry potassium fluoride in the form of powder was influencing pentachlorbenzonitrile in sulpholane or benzonitrile at 300-315 oC for 18 hours we can obtain pentafluorobenzonitrile with the yield higher than 85% [23]. The influence of potassium fluoride on tetrachlorophthalonitrile [24] or tetrachlorisophthalonitrile [25] at 220 oC produces corresponding terafluoroderivatives.
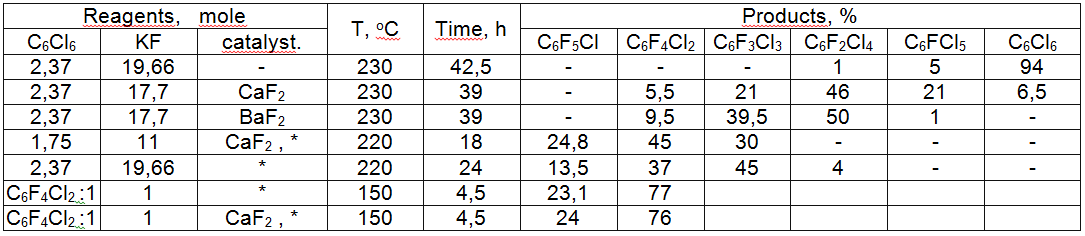
2,6-Difluorobenzonitrile is obtained by potassium fluoride influencing 2,6-dichlorobenzonitrile in dimethylsulphoxide (190 oC, 3 h) with the yield of 69 % [25]. The main reason of low yield and need to work at high temperatures is caused by low solubility of potassium and cesium fluorides in polar aprotic solvents like sulpholane, N-methylpyrrolidone, dimethylformamide. That's why Halex methodology works well only in case of activated benzene derivatives and is hardly suitable for temperature unstable substrates. Besides that, the isolating of target product is often hard to be carried out, at that ecologically unsafe solvents don't transform at all and lead to unnecessary waste. A successful carrying out of chloroaromatic compounds fluorodechlorinating using potassium fluoride depends on several factors. Among them the most important things are the state of potassium fluoride crystal structure and substrate own reactivity. Potassium fluoride was tested using example of chlorine exchange for fluorine in benzene ring, containing NO2 group. It was prepared by different methods: obtained by high-temperature drying and spraying of aqueous KF solution (fine-dispersed powder) [26], dispersed onto neutral CaF2 [27], re-crystallized out of methyl alcohol [28], drying and freezing [29]. In all of the cases we have a great increasing of KF specific surface due to decreasing of crystal sizes, that in its turn result in noticeable grow of reactivity in reactions of nucleophilic substitution of aromatically bonded chlorine for fluorine. At that the solvent can play the main part. The authors of work [30] carefully studied the influence of potassium fluoride quality on chlorine exchange for fluorine in hexachlorobenzene, going without solvent and at high temperature. They used inert undercoats: potassium fluoride and barium fluoride. In table 2 you can see the results of the research. When using dried by spraying potassium fluoride the summary yield of fluorination products doesn't exceed 6%, while the application of potassium fluoride, put over calcium fluoride in comparable conditions exceeds the yield of fluorination products: tetrachlorodifluorobenzene was obtained with the yield of 46%. In case of using potassium fluoride put on barium fluoride we get almost the same results. Table 2. Interaction of Potassium Fluoride and Hexachlorobenzene (230oC, 42.5 h) [30]. * tetrakis(diethylamino)phosphonium bromide In spite of the successful putting of this process into production, the development of poly-fluoroaromatic compounds chemistry was suppressed by the imperfection of poly-chlorobenzenes potassium fluoride fluorination technology (Halex process), and by the its high inputs. Due to these circumstances, the researches regarding search for new and upgrading the known obtaining method of hexafluorobenzene itself continued and in recent years the same researches regarding obtaining of octafluoronaphtalene also have begun. Here we mark out several main directions: 1. The upgrading of hexafluorobenzene obtaining method based on finding catalysts of inter-phase transfer, preparation of potassium fluoride with highly developed surface and application of high-boiling aprotic solvents. 2. The application of physical effects to speed up the exchange process of chlorine for fluorine and to lower the reaction temperature. 3. Using of fluorinating processes of poly-haloidbenzenes using elemental fluorine and other reagents followed by further dehalogenation under the metal's influence. 4. Fluoroaromatic compounds synthesis out of commercial chladones and polyfluorolefines. 5. Using fundamentally new solvents or systems of ionic liquids type. Seeing a main task and subject of chemistry as studying of compounds' transformations during chemical processes we'll make an attempt to concentrate our general attention on synthesis of key compounds of poly-fluoraromatic row that is hexafluorobenzene and octafluoronaphtalene, and on discussing methods of intensification of a known process and on development of new approaches. Simultaneously we have paid our attention to a most important obtaining method of not only low-fluorinated benzenes, but also of polyfluorinated ones, based on Halex-process. Obtaining of low-fluorinated aromatic compounds by fluorinating of arenas, containing chlorine atom in positions, activated for example by NO2 group, is rather perspective in terms of creating pilot scale productions of a number of functional low-fluorinated arenas. Both parts are united by a common approach based on the exchange process of chlorine for fluorine under the alkali metals' influence. |
Fluorine Notes, 2006, 44, 1-2