Fluorine Notes, 2005, 43, 3-4
Characteristics and Application of perfluoropolyesteracids fluoroanhydrides.
B. Maximov, V. Kornilov
1. Perfluoropolyesteracids' fluoroanhydrides characteristics are the same as perfluoroacids fluoroanhydrides: thus they easily react with alcohols, amines and other nucleophilic agents forming esters, amides etc. respectively with yield close to quantitative one. Interaction reactions of acids perfluoropolyesters fluoroanhydrides and fluorolefines are of interest from a theoretical point along with quite famous characteristics.
It is known, that in the presence of alkali metals fluorides (KF, CsF, RbF) in aprotic solvents perfluoracids' fluoroanhydrides interact with hexafluoropropylene forming perfluoro(isopropylalkylketones) at temperature about 160-170 oC (R.D.Smith, F.S.Fawcett and others, J.Am.Chem.Soc, 81, 4285,(1962).
Perfluoropolyesteracids fluoroanhydrides together with alkali metals' fluorides form alcoholates (without using of aprotic solvents) at ~40 oC.
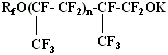
Such a high reactivity of perfluoropolyester acids' fluoroanhydrides makes it possible to carry out their reaction with hexafluoropropylene in the presence of HF and aprotic solvent at 20 oC with quantitative yield of perfluoropolyesterketones:
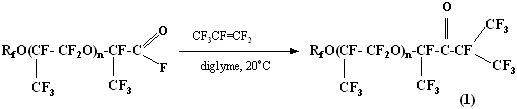
In alkali medium such ketones are subject to haloformic decomposition forming monohydrooligoether and pefluoroisobutyric acid (its salt):
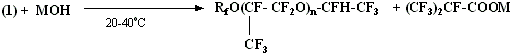
During UV-radiation and at high temperature in compound (1) the carbonyl group is eliminated forming compounds:
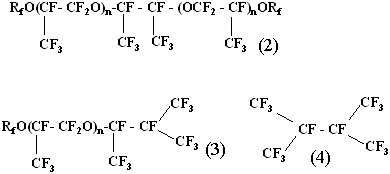
At temperature below 80 oC compound (3) is formed in the majority of cases, at temperature above 120 oC the compounds (2) and (4) are mainly formed.
The above example of interaction between perfluoropolyesters' fluoroanhydrides and hexafluoropropylene and following transformations of compound formed under the influence of UV gives a great opportunities regarding synthesis of various fluoroorganic compounds, using different fluorolefines in the reaction.
2. The presence of oxygen atoms in fluoroalkyl chain increases its flexibility and conformational abilities, what leads to increasing of surface activity of such surfactants. Perfluoropolyester acids' derivatives (0,1% of mass and less) lower surface tension of aqueous media to 16-18 mn/m. Numerous derivatives of perfluoropolyester acids of common formula RFZP possess these properties:
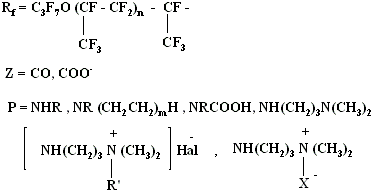
H, K, Na , NH4 at Z=COO-
R' = CH3, C2H5, C2H4OH
X= CH2COO-
Hal = Cl, Br, I
n = 0 - 2 , m = 1 - 30
Perfluoropolyester acids derivatives find their wide application in different fields of technics, particularly:
- As effective wear-preventive additives for engine oils due to formation a fine preventive coating on wear joints, - a monomolecular layer of fluorine surface-active material (50-60 angstrem), possessing high antifriction properties. This allows to increase friction assemblies resource, to improve starting characteristics of engines, to decrease the fuel consumption etc.
- During covering of the solid surfaces (metals, mechanical rubber articles, polymers etc.) with fluorine surface-active material monomolecular films based on derivatives of perfluoropolyester acids they abruptly change their physical chemical and energetic properties. Thus surface energy of metals and mechanical rubber articles decreases down to 4-6 mn/m, In consequence of what the limiting wetting angle is increasing (for water up to 100, for oils - up to 60-80), that is preventing lubricant from flowing out of friction assemblies
The application of different compositions containing fluorine surface-active material based on perfluoropolyester acids to treat different metal-working instruments such as drills, cutters and also multipurpose stamping equipment is based on the same effect of decreasing of surface energy.
The production technology of a number of compositions containing fluorine surface-active material based on perfluoropolyester acids derivatives of different purposes for treatment of friction assemblies, metal treatment, fire extinguishing etc. was invented in RSC "Applied Chemistry" according to listed above directions and some other of them.
Perfluoropolyester acids' derivatives decreasing the surface tension of liquids can be used in compositions for extinguishing of burning petrochemicals, polar liquids, alcohols. Such compositions not only form a barrier film on the surface of burning liquid but also have a foaming effect.
3. Fluoroanhydride group of perfluoropolyester acid eliminates itself at interaction with elemental fluorine or high fluorides of elements (CoF3, CeF4, SbF5 etc.)
Elimination reaction goes according to the following scheme:
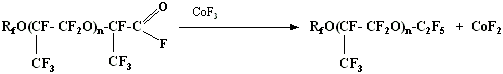
The yield of neutral polyesters is about 85-95% when using elemental fluorine as fluorinating agent at 170-200 oC, when using CoF3 - at 140-160 oC, when using SbF5 - at 70-90 oC.
Neutral (stabilized) perfluoropolyesters are a mixture of compounds of molecular mass ranging from 800 to 104 , viscosity from 10 to 2000 cct at 20 oC, density within 1750-1920 kg/m3, vapour pressure about 10 -3 - 10 -14 torr at 20 oC, freezing point within the range of minus 80-110 oC to minus 20 oC.
Average distribution of molecular masses:
~800 = 7-15%, ~1000 - 1500 = 35 ? 60%,
~2500 = 15 - 20 %, more 10.000 ~ 15 %.
Perfluoropolyesters' properties and distribution of molecular masses are determined by conditions of carrying out of co-polymerization process between hexafluoropropylene and oxygen.
High stability of C-O, C-C, C- F bonds in perfluoropolyesters, chains' flexibility, small amount of interchain interaction strength determines the appearance of complex of important properties for their application in technics: their high thermal and chemical stability combines with good low temperature characteristics (congelation point reaches 110 oC).
All that allows wide using of perfluoropolyesters as thermal and chemical stable oils, lubricants and liquids in the field of technics. Stabilized perfluoropolyesters are resistant to strong acids and bases even at high temperature (~100 oC), they do not change their physical and chemical properties at radiation influence of dose of radiation power up to 108 - 109 rad.
An important feature of hexafluoropropylene (and other fluoromonomers) and oxygen co-polymerization method is the modification opportunity of perfluoropolyester acids' fluoroanhydrides obtained at the first stage using different fluoromonomers CF2 =CF- RF (above we have a hexafluoropropylene example) with further elimination of carbonyl group and obtaining of compounds of type (5) :
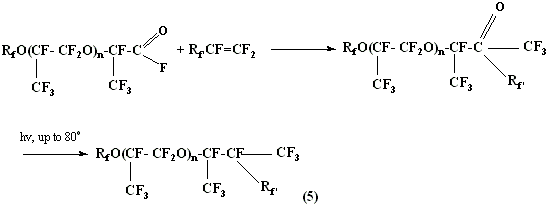
Perfluoropolyethers structures (5) are of interest because of the fact, that at different R'F higher decompositions points can be reached, and lower freezing points ( for more than 20 - 30 oC ) may be reached compare to linear perfluoropolyesters at almost equal molecular mass.
This effect allows essential widening of perfluoropolyesters synthesis opportunities and their wide temperature application range.
Fluorine Notes, 2005, 43, 3-4
