2. Maleic acid fluorine containing mono- and diesters obtaining.
3. The synthesis of sulfoacids derivatives based on fluorine containing acrylic and
maleic acids esters and their salts.
4. Polymerization and co-polymerization of fluorine containing acrylates.
5. Practical application fields of fluorine containing polymer materials based on
fluorinated acrylates and maleates.
Introduction
At every stage of technical progress the role and direction of fundamental researches,
especially the ones of new class of organic compounds is initiated by society's
need in new materials, which can't exist without creating fundamentally new,
possessing high consumer properties and able to work under more severe conditions
materials. Indeed fluorine compounds meet such requirements and can play
a defining part in intensification and production simplification of many
goods without fundamental reconstruction of existing manufactures. Perfluorinated
compounds are the compounds of our present and future. The production of
such perfluorinated organic compounds has already been developed. Such compounds
meet up-to-date requirements by thermophysical and dielectric characteristics,
they can be used in a wide temperature and thermal loads ranges their practical
application field is also found. The entire replacement of hydrogen atoms
for fluorine in the organic molecule results in sharp changing of properties,
what has been used to create new generation materials possessing excellent
working characteristics. They began to be used for metals and alloys atmospheric
and salt corrosion defense, and also as lubricating oils, applied at aggressive
conditions, in hydraulic liquids, as heat-transfers, liquids for vacuum pumps,
operating in corrosion active medium, as additives for oils, used at high
pressure in different compressors etc. In terms of scope and level of scientific
achievements and scale of their commercial implementation the fluorine chemistry
is now a powerful independent scientific and technical direction of organic
chemistry.
The production of fluorine containing materials doesn't only depend on the level
of our knowledge regarding fluororganic compounds properties, but also on
their practical application perspective, which is dictated by development
of technical conception.
Among fluoropolymers [1-3] an independent and intensively developing class of
polymers and co-polymers, acrylates and methacrylates [3-7] fluorine modified
fills an important place. It occurs because of the fact, that acrylates based
on fluorinated alcohols posses unique properties, especially high hydro-
and oleophobic abilities. It makes a base to obtain polymeric suspensions
for fibrous articles surface proccessing and creating coatings for non-organic
materials. In most cases water and oil-repellent agents are obtained out
of polymers, which main chains are poly-acrylic and polymetacrylic acid,
turning acid groups into fluorocarboxylic chains ester ones (C8-C10) [8-12].
They are surfactants, sorbing at the edge of phase break sharply change surfaces'
nature and properties. Their specific surface activity, which can't be reached
in case of hydrocarboxylic component, made a base to create materials, which
achieved a strong position among commercial products. Surface energy of coatings
made of such fluorine containing polymers is low, and that's why they can
be hardly dampened and are characterized by low friction coefficients comparatively
to other solid materials. All said above allows creating materials for field
of technics. The field of their practical application includes different
directions. For example, we can point out the water and oil-repellent properties
adding chemicals for fabrics [13], materials for microelectronics [14], surfactants
(SAM) for olefin's polymerization processes [10], systems stable to electronic
strike and X-rays [15-17] etc.
Here we see the chemical enterprises re-orientation to science intensive articles
production, new technologies and new products.
The synthesis of fluorine modified monomers (acrylates) is mainly carried out
by interaction of acrylic and metacrylic acids with linear partially fluorinated
alcohols, that was boosted by patent claim [18]. The interest for modified
acrylates is constantly growing a great file of information on this class
compounds, especially in the field of practical application. If earlier at
acrylates synthesis they used linear poly-fluorinated aliphatic alcohols,
then by now the interest for introduction opportunity of hydophilic fragments
such as ether bounds, nitrogen atoms etc into main chain of molecule is shown.
Such opportunity for acrylates can be implemented in hydrocarboxylic skeleton
of ester part.
Acrylic and metacrylic acids are commercially produced in huge amounts. Because
of this the researchers paid a lot of their attention to developing of partly
fluorinated alcohols obtaining methods and technology and other unsaturated
organic compounds co-polymeriazation proccesses [1,3]. The requirements for
properties of polymer materials used for techniques, in regards to wearing
qualities, weather resistance, incombustability, antiadhesive and anti-corrosive
properties, dielectrical and other special characteristics remain high. Owing
to so strict requirements for such materials and in spite that their production
is complicated and price is high compare to other plastics they work up the
market. Combination of fluorine containing polymer materials based on acrylates
improves characteristics of chemical and atmosphere stable coatings.
In this review we demonstrate the last decade achievements in the field of synthesis
and application of polymer materials based on acrylates and maleates, containing
fluorine modified ester fragments, synthesis of which is based first of all
on use of partly fluorinated alcohols. These alcohols production technology
is based on the following chemical transformations. .
1) Alcohols like RFCH2CH2OH
are produced out of RFI perfluoroalkyliodides, which at first
are introduced into reaction with ethylene with further saponification of
new sub-derivative [19,20].
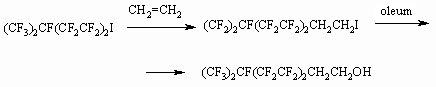
The method is implemented in commercial scale.
2) Synthesis method of H(CF2CF2)nCH2OH
(n = 1-15) telomeric alcohols is based on telomerization of tetrafluoroethylene
and other fluorolefines in methyl alcohol or aliphatic alcohol in presence
of peroxide initiators [21-55].
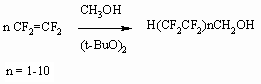
This process is implemented commercially.
3) Alcohols like RFCH2OH are obtained
by reduction of polyfluorinated carbonyl containing compounds (perfluorocarboxylic
acids ethers [51,56-69] and diketons and ketofluoroanhydrides [70]) by NaBY4 or LiAlH4 action.
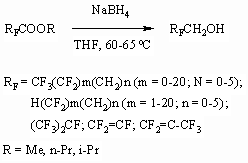
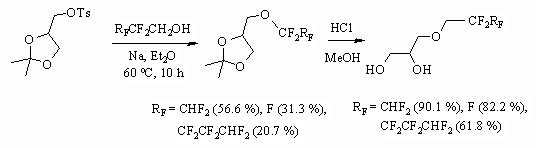
4) Synthesis of diols with polyfluoroalkyl groups was developed
by Paleta and his co-workers. It is carried out by reaction of partly fluorinated
alcohols and 4(hydroxymethyl)-2,2-dimethyl-1,3-dioxolane tosylate with further
deprotonation of reaction product [71-79].
Acrylic acid itself and methacrylic acid or its chloroanhydride, anhydride, maleic
acid or its anhydride are used as unsaturated carboxylic acids.
1. Obtaining of Fluorine Modified Acrylates Ester Fragment.
Esterification of acrylic and methacrylic acid by poly-fluorinated alcohols like
R(CF2CF2)nCH2OH (R = H, F; n
= 1-4) or F(CF2CF2)nCH2CH2OH
(n = 1-4) is carried out in presence of acidic catalyst sulfocoal and concentrated
sulfuric acid in toluene medium at 110 oC for 6-8 h [80-83]. 2-perfluoroalkylethyliodides
like (CF3)2CF(CF2)4CH2CH2I
can also be used, but in this case acrylic acid potassium salt is used and
reaction is held in presence of C6H13N+Et3 .I- salt in isopropyl alcohol at 120 oC
for 5 h [84].
Fluorine containing acrylate synthesis can be carried out without isolating of
corresponding alcohol. Thus, the authors of the work [85] showed such an
opportunity, using two apparatus. Inside the first one the tetrafluoroethylene's
telomerization is carried out in the presence of ethyl iodine, then reaction
products are put inside the second apparatus, where the acrylic acid and
tert-butyl alcohol are added, that leads to formation of corresponding acrylate.
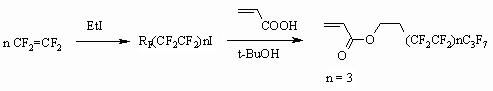
Most often acrylic and methacrylic acid chloroanhydride and anhydride are used,
the reaction is carried out in the presence of Et3N.
When H(CF2CF2)nCH2OH ( n = 1-4) linear
fluorinated alcohols are influencing acrylic acid chloroanhydride in the
presence of bases (KOH, NEt3) acrylates are formed in acetonitrile
(1a-d) [30,80,81]. Here the base plays the definitive part.
Thus when using potash (K2CO3) in acetonitrile we get
corresponding ester of acrylic acid 1a in the mixture of
initial alcohol; NEt3 promotes the forming of only one product
1a, while KOH is very effective, however we get not only
the 1a product we expected but also (2a)
compound, which is a result of fluorinated alcohol addition according to
C=C bond of reaction product 1a. It should be noticed that
before was showed [86] the formation of addition product according to C=C
bond of telomeric alcohols with acrylonitrile, though those authors carried
out their reaction in the presence of sodium metal at 40 oC (yield
is 73 %). At present conditions with acrylic acid chloroanhydride other telomeric
alcohols along with 1b-d acrylates produce also 2b,c compounds.
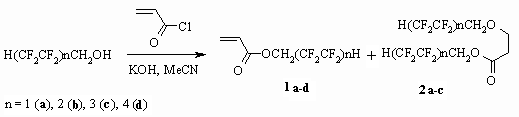
Thus, depending on fluorinated alcohol being used we should apply either triethylamine
or KOH as a base, that dependes on their activity. Other fluorinated alcohols
3a-g which have a branching in carbon chain especially in
the beginning, or oxygen atoms are introduced into reaction with acrylic
acid anhydride, at that acrylates 4a-g were obtained [30].
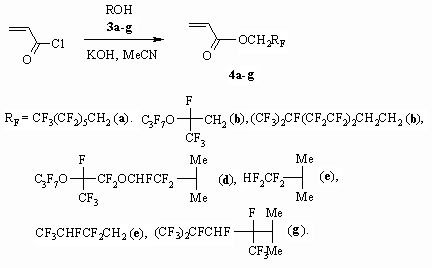
As the 3g alcohol used had an admixture of isomeric alcohol
(11 %) the 4g acrylate formed is also a mixture of two isomers
with the same composition. In case of 3d alcohol (mixture
of diastereomers of 1:1.65 proportion) the formed 4d acrylate
is also obtained as a mixture of two diastereomers, which proportion is 1:1.1
(according to chromato-mass-spectrometry data and NMR 1H and 19F
spectra).
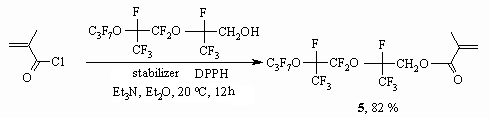
By the example of 3d alcohol reaction they show the opportunity
of methacrylic acid chloanhydride introducing into reaction forming the corresponding
methacrylate 5 [58].
Alcohols with spatially branched carbon chains are also effective for the acrylates
synthesis [87,88]. You can look for the review on 2-phenyl-1,1,1,3,3,3-hexafluoropropan-2-ol
using for polyacrylates synthesis in [89].
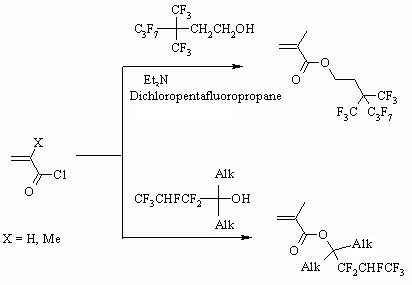
Esterification of methacrylic acid using secondary fluorocontaining alcohols
in the presence of sulfuric acid isn't very much effective, that's why it
is more preferable to use chloroanhydride or methacrylic acid anhydride.
Thus, morpholine fluorinated metacrylate is obtained with high yield when
corresponding alcohol reacts with the methacrylic acid anhydride [60].
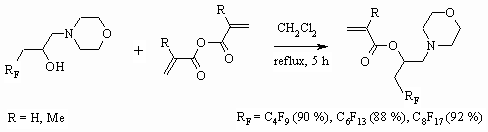
Secondary alcohols like F(CF2)nCH(CH2Cl)OH (n
= 6,8,10) react with acrylic acid choloanhydride in the presence of Et3N
in benzene at room temperature for 2 hours forming the corresponding acrylate
with high yield [90].
1-(Polyfluoroalkyl)ethan-1,2-diols produce corresponding bis-metacrylates [73]
when methacrylic acid chloroanhydride influence them. We should note, that
process conditions essentially influence the proportion of monoester and
diester. Thus, using the medium of pyridine, diethyl ether and boiling for
72 h- produces solely monoether [74], while in the system of triethylamine,
diethyl ether, room temperature and 2 h time of reaction we get solely diether
[73].
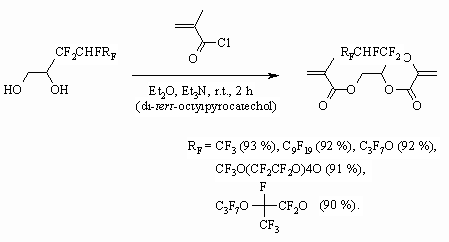
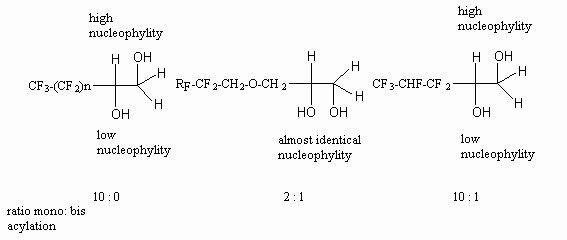
The authors of work [71] point out an important role of process conditions for
the conversion level of fluorocontaining 1,2-diol with methacrylic acid chloroanhydride.
Initially only one of the two -OH groups of fluorocontaining 1,2-diol reacts.
Perfluorinated group influences its reactivity. The authors of work [71]
have established the following row of activity for some of 1,2-diols.
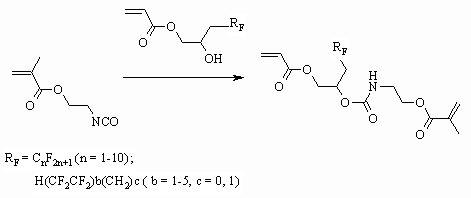
Other esters of methacrylic acid also react with 1,2-diol having perfluoralkyl
group [91-93] or with perfluoropolyether [94], at that the compounds, having
acrylic and urethane fragments are formed.
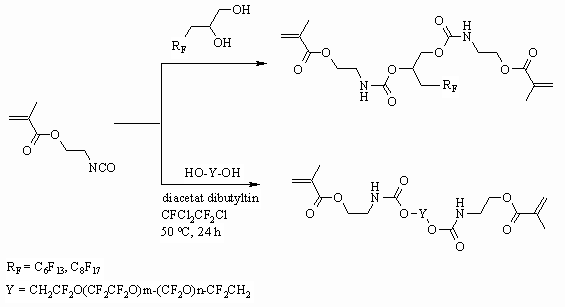
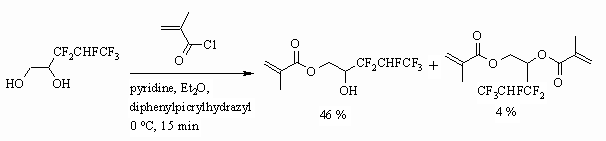
Monoester, obtained by interaction of fluorocontaining 1,2-diol and acrylic acid
chloroanhydride reacts with 2- methacrylethylisocyanate and as a result we
get a compound with acrylates and urethane functions [95,96].
The authors of work [97] showed the opportunity of acrylates obtaining using
perfluorolefines according to the following scheme and without using fluorinated
alcohols.
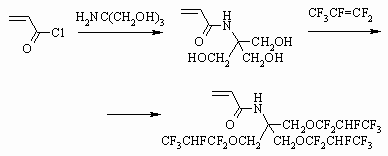
 -fluoroacrylic acid fluoroanhydride,
which is transformed into esters by reaction with telomeric alcohols[98-101],
is obtained by interaction of 2,2,3,3-tetrafluoroxetane, alkali metals halogenides
and dehalogenizing agent in aprotic bipolar solvents in the presence of radical
polymerization inhibitor.
-fluoroacrylic acid fluoroanhydride,
which is transformed into esters by reaction with telomeric alcohols[98-101],
is obtained by interaction of 2,2,3,3-tetrafluoroxetane, alkali metals halogenides
and dehalogenizing agent in aprotic bipolar solvents in the presence of radical
polymerization inhibitor.
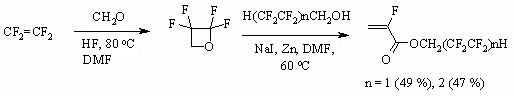
Polymer materials based on fluorine modified acrylates and methacrylates according
to ester fragment are of great interest of waveguides and optical glassfiber
production because they are high transparent in the range of 1200-1600 nm,
what is used for optical connection, along with their relatively low refracting
indexes [102-104]. These properties can be enhanced by introducing fluorine
atoms or other heavy atoms into carbon chain. Modified derivatives of initial
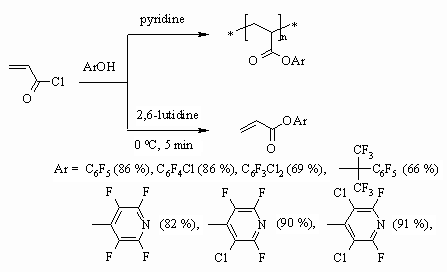
monomer are of interest, they are obtained by introducing pentafluorophenyl
group into eater part of molecule. The works [105,106], during which authors
had carried out acrylates' synthesis using fluorine containing phenols, are
devoted to this subject. It turned out, that a base used plays an important
part. Thus in case of pyridine application the acrylate forming reaction
goes together with polymerization process, while in the presence of 2,6-lutidine
we get only acrylic acid ester.
The reactions of pentafluorophenol with 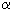 -chloroacrylate,
-chloroacrylate, 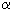 -fluoroacrylate and metacrylate
were earlier described in the [102-104].
-fluoroacrylate and metacrylate
were earlier described in the [102-104].
To be continued
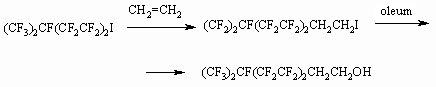
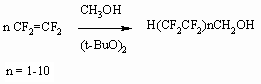

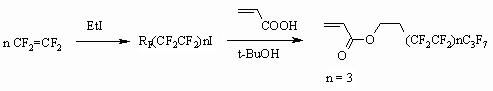
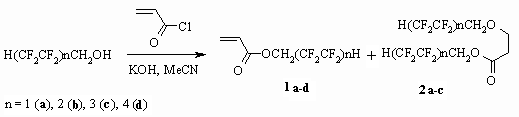



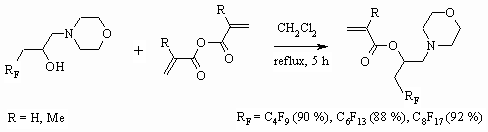
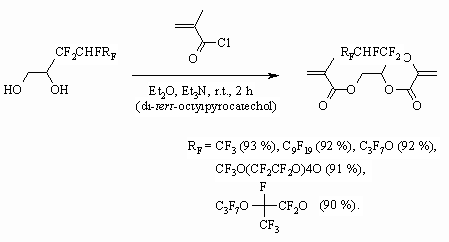


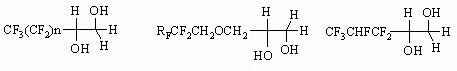



 -fluoroacrylic acid fluoroanhydride,
which is transformed into esters by reaction with telomeric alcohols[98-101],
is obtained by interaction of 2,2,3,3-tetrafluoroxetane, alkali metals halogenides
and dehalogenizing agent in aprotic bipolar solvents in the presence of radical
polymerization inhibitor.
-fluoroacrylic acid fluoroanhydride,
which is transformed into esters by reaction with telomeric alcohols[98-101],
is obtained by interaction of 2,2,3,3-tetrafluoroxetane, alkali metals halogenides
and dehalogenizing agent in aprotic bipolar solvents in the presence of radical
polymerization inhibitor.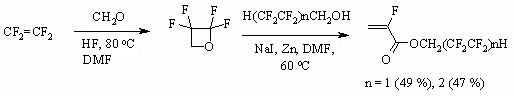

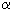 -chloroacrylate,
-chloroacrylate, 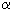 -fluoroacrylate and metacrylate
were earlier described in the [102-104].
-fluoroacrylate and metacrylate
were earlier described in the [102-104].