Fluorine Notes, 2005, 40, 1-2
ACRYLIC ACID CHLOANHYDRIDE AND FLUORINATED ALCOHOLS INTERACTION AS A BASE FOR FLUORINE MODIFICATION OF ACRYLATES.
P.A. Podsevalov*, G.G.Furin**
* PIKO LLC, Perm City, Russia
** Novosibirsk Institute of Organic Chemistry named after N.N. Vorojtsov Siberian Department of RAS
Here we discuss the approach of fluorine modification of acrylates' esters fragment using for their synthesis partly fluorinated alcohols , they are obtained by reduction poly-fluorocarboxylic acids esters, containing oxygen atoms in carbon skeleton and possessing secondary and tertiary carbon atoms. Acrylic acid poly-fluorinated esters are obtained by interacting appropriate partly fluorinated alcohols and chloroanhydrides of acrylic and meta-acrylic acids . Acrylates' polymerization is carried out using radical initiators forming low-molecular polymers, which dissolve in organic solvents.
Acrylates based on fluorinated alcohols posses unique properties, allowing to create materials for new field of technics. The field of their practical application covers different directions. For example, we can mention the chemicals, which make the materials water and oil-repellent [7], materials for microelectronics [8], surface-active materials for the olefins polymerization process [4], electronic strike and X-rays high resistant systems [10,11] etc. If at first stage at acrylates' synthesis we used linear poly-fluorinated aliphatic alcohols, then for now the interest is shown to branched carbon fragment and carbon skeleton, containing oxygen atoms, and to ether part.
The synthesis of ester fragment fluorine modified acrylates is carried out using the following linear poly-fluorinated alcohols:
1) RFCH2CH2OH that are obtained based on perfluoroalkyl iodides RFI, which are introduced into ethylene reaction with further saponification of new iodo-derivatives [12,13].
2) Telomeric Alcohols H(CF2CF2 )nCH2OH (n = 1-15) that are obtained by telomerization of tetrafluoroethylene in methyl alcohol in the presence of peroxide initiators [14-16].
3) Alcohols RFCH2OH that are obtained by reduction of perfluorocarboxylic acids esters [17-19].
The purpose of the present work was the synthesis of new acrylates, which is based on partly fluorinated alcohols, obtained by reduction of commercially produced perfluorocarboxylic acids' esters, and disclosing their properties.
We showed, that methyl ester of perfluoroheptanoic acid and acids based on tetrafluoroethylene oxide
trimer and hexafluoropropylene oxide dimer are reduced smoothly by NaBH4 action
in diethyl ether or monoglyme (diagram 1). Thus, 1H, 1H- perfluoro-3,6,9-trioxadecan-1-ol (I b)
is obtained according to this method with the yield of 82% and alcohol (I c) is
obtained analogously to described for methyl ester of acid out of hexafluoropropylene oxide trimer
with the yield of 91% [19].
Diagram 1
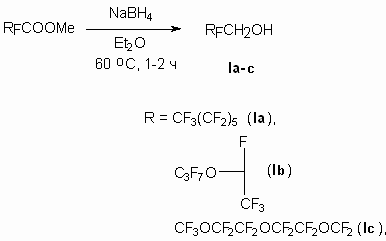
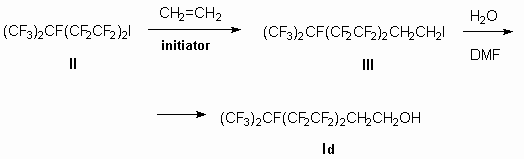
Starting from iodide (II) according to the famous literature diagram polyfluoroalkyliodide
(III) is obtained by ethylene action, then transferred into alcohol (IV)
by interaction of the last mentioned with water in the dimethylformamide medium (diagram 2).
Diagram 2
Alcohols (Ie-i) are obtained according to the reaction [20] of the corresponding olefins with methyl and isopropyl alcohols in the presence of di-tert-butylperoxide, which are used to obtain acrylates (see below).
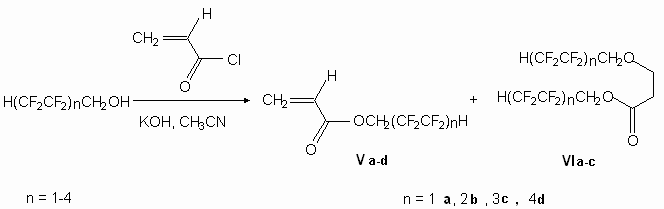
When linear fluorinated alcohols H(CF2CF2)nCH2OH (IV n = 1-4 : a-d ) are acting upon acrylic acid chloroanhydride in the presence of bases (KOH, NEt3) acrylates (Va-d) are formed in acetonitrile. Here the base has a defining role. Thus when we use potash (K2CO3) a corresponding ester of acrylic acid (Va) is formed in acetonitrile together with starting alcohol; NEt3 forwards the forming of just one product (Va), while KOH is very effective, however we get not only the product we've expected (Va), but also the compound (VIa), which is a result of fluorinated alcohol addition according to bond reaction product C=C (Va). Earlier it was showed [21] the formation of addition product according to C=C bond of telomeric alcohols with acrylonitrile, though that authors conducted the reaction in the presence of metallic sodium at 40 oC (yield = 73 %). We have showed, that other telomeric alcohols at present conditions with acrylic acid chloroanhydride produce along with actylates (V b-d) also compounds (VIb,c) (diagram 3).
Diagram 3
Thus, depending on fluorinated alcohol used we should use either triethylamine or KOH as a base, it depends on their activity. Other fluorinated alcohols having a branching in carbon chain especially in its beginning or oxygen atoms were introduced into reaction, at we got acrylates (VIIa-i) (diagram 4).
Diagram 4
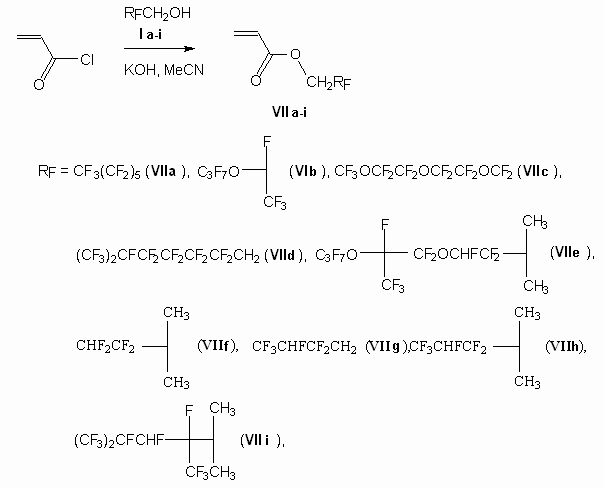
As the alcohol used (I i) had an admixture of isomeric alcohol (11 %) the acrylate formed (VII i) also was a mixture of two isomers with the same composition. In case of (Ie) alcohol (mixture of diastereoisomers with proportion of 1:1.65) the resulting acrylate (VIIe) is also obtained in the form of mixture of two diastereoisomers , which proportion counts to 1:1.1 (according to chromato-mass-spectrometry data and NMR 1H and 19 F spectras).
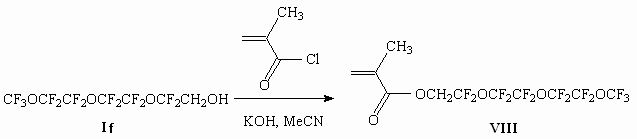
Here using example of alcohol (I f) reaction we showed the opportunity of methacrylic acid chloanhydride introduction into reaction too (diagram 5).
Diagram 5
The structure of products obtained is confirmed by IR, NMR 1H, 13C and 19F spectras data and mass-spectrometry. In the NMR 1H spectras of synthesized partly fluorinated compounds the protons' signals of CH2=CHC(O) group are presented, which sizes of chemical shifts are typical for such kinds of hydrocarbon analogues (table 2). In NMR 19F and 13C spectras we observe the signals of fluorine and carbon atoms which sizes of chemical shifts and spin-spin coupling constants are the same for CF3CHFCF2-, -OCH2CF2CF2- groups, moreover for fluorine atoms of CF2 (CF3CHFCF2-) group the signals' structure is an AB-system with JFF 140-280 Hz. A signal from fluorine atom of CHF (CF3CHFCF2-) group is located in a strong field -40 - -49 ppm.
Acrylates (Va-d) - (VIII) under the action of radical initiators in aqueous solutions of acetone are polymerized forming polymers, which are soluble in organic solvents. This allows to obtain thin films at different materials.
Thus, partly fluorinated aliphatic alcohols can be effective O- nucleophilic reagents, what widens their practical application field of synthesis of different fluorine containing materials.
Experimental Part
1H, 13C and 19F NMR spectras were recorded at Bruker WP 400 SY spectrometer at frequencies of 400, 100, 188 MHz respectively ( internal standards HMDS, C6F6 ( JCH were not measured) and CDCl3). IR-spectras (5 % in CCl4) were recorded at spectrometer Specord M-80 (CCl4); chromato-mass-spectras (power of ionizing electrons is 70 eV) were recorded at Finnigan MAT-8200 and mass-selective detector chromatograph (Hewlett Packard G 1800 A GCD). In the last case we used the column of 30 m, 0.25 mm diameter, coated inside with layer of co-polymer 5% diphenyl- 95% dimethylsiloxane (HP-5) (thickness is 0.25 mkm). Gas-carrier is helium, 1 ml/min, evaporator temperature is 280 oC. The column temperature was increased from 50 (hold for 2 min) to 280 oC (hold for 5 min) at a rate of 10 degree/min. Control of all reactions was carried out using NMR 19F method. Reaction mixtures were analyzed at chromatograph LHM 72 (15 % SE-30, CKTF-803, QF-1, chromosorb W, column of 4000 mm, 4mm diameter). You can find analytical data and compounds' characteristics in table 2.
The Formation of Partly Fluorinated Alcohols at fluorocarboxylic acid
ester reduction
8.2 g (0.205 g-mole) of NaBH4 and 100 ml of
diethyl ether were loaded into 3-neck flask equipped with effective cooler, stirrer and dropping
funnel. Then stirring we added 68.8 g (0.2 g-mole) of methyl 2,2,3,3,4,4,4,-heptafluorobutyrate in
solution of 5ml of methyl alcohol and 50 ml of diethyl ether. After reaction mixture was being heated
at boiling for 12 hours while stirring, cooled and 50 ml of hydrochloric acid were added (dilution
of concentrated one in proportion 1:1), the organic layer was isolated and extracted using diethyl
ether out of aqueous one. Extracts were integrated and dried over MgSO4. Mixture was filtrated
solvent was evaporated, residue was fractionated. We got 60. 5 (92 %) of 2,3,3,3-tetrafluoro-2-(heptofluoropropoxy)propan-1-ol
(I b), b.p. 83-84 oC (20 mm Hg).
Analogously we carried out
the synthesis of fluoroalcohols reducing corresponding carboxylic acids' methyl esters (Ia,c)
.
The Reactions of Partly Fluorinated Alcohols with Acrylic Acid Chloroanhydride
At room temperature the 23.2 g (0.1 g-moles) of alcohol IVb were added dropwise while stirring to suspension of 6.7 g (0.12 g-moles) of KOH and 0.1 g of hydroquinone and to 75 ml of acetonitrile and all that was hold at this temperature for 1 hour. After that we cooled the reaction mixture with ice water and we were adding dropwise 9 g (0.1 g-mole) of acrylic acid chloranhydride during 20 min. The mixture was being stirred 1 hour at that temperature and then the temperature was increased up to 40 oC and hold for 1 hour. The reaction mixture was poured into water, the organic layer was isolated, washed off with water, dried over MgSO4 and distilled.
The first fraction which b.p. is 41-42 oC (6 mm Hg) and weight is 19.2 g (67 %) contained
2,2,3,3,4,4,5,5-octafluoroamyl ester of acrylic acid (Vb). IR-spectra
(CCl4, 5 %), ![]() (cm-1) : 3014, 2964 (C-H), 1750 and 1705 (C=O), 1636 (C=C), 1409
(C-O), 1288 and 1248 (acrylate), 1201 (C-O), 1174 and 1134 (C-F), 982 and 900 (acrylate). Mass-spectra,
m/z (Irel, %) : 286 [M]+ (3.01), 267 [M-F]+ (5.82), 195 [CF2CF(CF2)2CH2
]+ (3.19), 145 [CF2CFCF2CH2 ]+ (5.23), 131 [CF2CFCF2 ]+ (6.56),
100 [CF2CF2]+ (3.20), 85 [CH2CHCOOCH2]+ (25.07), 69 [CF3]+ (12.71), 56 [CH2CHCO]+ (100), 51 [CHF2]+ (32.05), 31 [CH2OH]+ (66.25), 29 [HCO]+ (10.17),
27 [CH2CH]+ (45.81).
(cm-1) : 3014, 2964 (C-H), 1750 and 1705 (C=O), 1636 (C=C), 1409
(C-O), 1288 and 1248 (acrylate), 1201 (C-O), 1174 and 1134 (C-F), 982 and 900 (acrylate). Mass-spectra,
m/z (Irel, %) : 286 [M]+ (3.01), 267 [M-F]+ (5.82), 195 [CF2CF(CF2)2CH2
]+ (3.19), 145 [CF2CFCF2CH2 ]+ (5.23), 131 [CF2CFCF2 ]+ (6.56),
100 [CF2CF2]+ (3.20), 85 [CH2CHCOOCH2]+ (25.07), 69 [CF3]+ (12.71), 56 [CH2CHCO]+ (100), 51 [CHF2]+ (32.05), 31 [CH2OH]+ (66.25), 29 [HCO]+ (10.17),
27 [CH2CH]+ (45.81).
Second fraction which b.p. is 119-120 oC (6 mm Hg) weight is 7.9 g (15.8 %) contained 2,2,3,3,4,4,5,5-octafluoroamyl ester of
3-(2,2,3,3,4,4,5,5-octafluoropropoxy)propionic acid (VIb). IR-spectra (CCl4,
5 %), ![]() (cm-1) : 2973, 2931 and 2890 (C-H), 1767 and 1738 (C=O), 1402 (C-O), 1175 and 1134 (C-F),
976 and 902 (C-O). Mass-spectra, m/z (Irel, %) : 498 [M-HF]+ (3.15), 475 (2.38),
317 [M-H(CF2)4]+ (14.37), 303 [M-H(CF2)4CH2 ]+ (13.93), 287 [H(CF2)4CH2OCH2CH2CO]+ (100), 215 [H(CF2)4CH2]+ (11.55), 145 [CF2CFCF2CH2]+ (60.87), 131 [CF2CFCF2 ]+ (5.29), 113 [CF2CF2CH2OH]+ (12.90), 95 [CF2CH2OCH3]+ (25.61), 71 [CH2CHCOO]+ (78.33), 69 [CF3]+ (37.56), 57 [CH2CHCOCH2]+ (27.05), 55 [CH2CHCO]+ (93.80), 51 [CHF2]+ (54.48).
(cm-1) : 2973, 2931 and 2890 (C-H), 1767 and 1738 (C=O), 1402 (C-O), 1175 and 1134 (C-F),
976 and 902 (C-O). Mass-spectra, m/z (Irel, %) : 498 [M-HF]+ (3.15), 475 (2.38),
317 [M-H(CF2)4]+ (14.37), 303 [M-H(CF2)4CH2 ]+ (13.93), 287 [H(CF2)4CH2OCH2CH2CO]+ (100), 215 [H(CF2)4CH2]+ (11.55), 145 [CF2CFCF2CH2]+ (60.87), 131 [CF2CFCF2 ]+ (5.29), 113 [CF2CF2CH2OH]+ (12.90), 95 [CF2CH2OCH3]+ (25.61), 71 [CH2CHCOO]+ (78.33), 69 [CF3]+ (37.56), 57 [CH2CHCOCH2]+ (27.05), 55 [CH2CHCO]+ (93.80), 51 [CHF2]+ (54.48).
Analogously we carried out reactions of acrylic acid chloroanhydride with fluoroalcohols,
resulting in acrylates' obtaining (Va-d) - (VIII) (table 2).
References
[2] Clark J.C., Newland J.C., Ramrath R.F., Burleigh M.B., Schaffer K.R. // Pat. U.S. 6613862 (2003). Treat of fibrous substrates to impart repellency, stain resistance and soil resistance. C08G018/79.
[3] Soane D.S., Offord D.A. // Pat. U.S. 6617267 (2003). Modified textile and other materials and methods for their preparation. B32 B 027/12.
[4] Yao Li // Pat. U.S. 6638610 (2003). Water and oil repellent porous materials and processes for making the same. B 32 B 003/26; B 32 B 027/14.
[5] Lui N., Podszum W. // Eur. Pat. Appl. EP 744394 (1996). Perfluoroalkenyl group-containing (meth) acrylates, their preparation and their use. Chem. Abstr. 1997. Vol. 126. 19428n.
[6] Moore G.G.I., Mc Cormick F.B., Chattoraj M., Cross E.M., Liu J.J., Roberts R.R., Schulz J.F. // Pat. U.S. 6005137 (1999). Halogenated acrylates and polymers derived therefrom.
[7] Fielding H.C. // in Organofluorine Chemical and their industrial Application. (Ed. R.E. Banks) Ellis Horwood : Chichester. 1979. P. 214.
[8] Bernar B., Devaty J., Kralicek J., Zachoval J. // Org. Coat. Appl. Polym. Sci. Proc., 1983. Vol. 48. P. 711.
[9] Yong T.-M., Hems W.P., van Nunen J.L.M., Holmes A.B., Steinke J.H.G., Taylor P.L., Segal J.A., Griffin D.A. // J. Chem. Soc., Chem. Commun., 1997, P. 1811.
[10] Bednar B., Marousek V., Zachoval J., Paleta O. // Pat. Czech. CS 273782 (1992). Polymeric electron and X-ray resists. Cl C 08 F 20/24. Chem. Abstr. 1993. Vol. 120. 120754s.
[11] Paleta O., Danda A., Stepan L., Kvicala J., Dedek V. // J. Fluorine Chem., 1989. Vol. 45. P. 331.
[12] Blochel W. // Pat. U.S. 3285393 (1970).
[13] Millauer H. // Pat. Ger. 2318677 (1975).
[14] Yamaguchi F., Takaki S., Yoshizawa T., Ogura E., Katsube // Pat. U.S. 6187969 (2001). Process for producing fluoroalcohol.
[15] Uklonskij I. P., Denisenkov V.F.‚ Il'in A.N., Ivanova L. M., Bahmutov YU.L., Mineev S.N. // Patent RU 2150459 (2000) Sposob polucheniya poliftorirovannyh spirtov MPK7 S 07 S 31/38
[16] Wada A., Tanaka H., Tanabe K., Yamagishi N., Toma T. // Appl. 1191009 EP (2002).Process for producing fluoroalcohol.
[17] Kawa H. // Pat. U.S. 6479712 (2002);
[18] Udagawa A. // Jpn. Kokai Tokkyo Koho JP 240571 (2001). Production method of fluoro alcohols by reduction of fluorinated esters using borohydride compound catalysts. Chem. Abstr. 2001. Vol. 135. 212588d.
[19] Paleta O., Palecek J., Michalek J. // J. Fluorine Chem., 2002. Vol. 114. P. 51-53.
[20] Il'in A. A., Ivanova L. M., Bahmutov Yu. L., Il'in A.N., Furin G. G., Pokrovskij L. M. // Zhurn prikl. khimii, v pechati.
[21] Kashkin A. V., Bahmutov Yu. L., Marchenko N. N. // Patent RU 316686 (1969). MPK7 S 07 S 121/38. Sposob polucheniyaβ-(poliftoralkoksi)propionitrilov.
Fluorine Notes, 2005, 40, 1-2
