Fluorine Notes, 2005, 38, 1-2
Perfluoropolyethers.
Report 2
prof. B. Maximov
RSC "Applied Chemistry" , S.-Petersburg, Russia, e-mail : boris_maximov@mail.ru
The oxidization of perfluoroolefines (hexafluoropropylene, tetrafluoroethylene) in the liquid phase using different initiation methods (UV-radiation or chemical compounds use (fluorine etc.) is the main commercial perfluoropolyethers' obtaining method.
Taking into account this fact we further will discuss only these two directions of perfluoropolyethers' synthesis. In this article you will find a short review of particularities of both methods. Hereinafter the main attention is paid to perfluoropolyethers' synthesis method fluorine initiated and also to the synthesis of perfluoropropylene oxides' derivatives obtained.
Photooxidation of Perfluoroolefines in Liquid Phase.
Hexafluoropropylene photooxidation in condensed phase at -60oC produces low-molecular compounds only in small quantities, there are mainly COF2 and CF3COF among them, and more than 90% of hexafluoropropylene transforms into viscous mixture of olygomers with the common formula
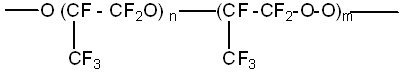
Using NMR F19 it is stated, that links are connected to each other mainly according "head to tail" type (1). The average polymerization degree (n+m) is 30-40.
The breakdown time of fluoroolefines' oxygen oxidization reaction is not long. If we add a small amount of peroxides there won’t be any breakdown time.
Tetrafluoroethylene oxidizes in the inert solvent solution (for example difluorodichloromethane, oxygen saturated (1,2) at atmospheric pressure because of its tendency to polymerize.
At that the main synthesis' products were CF2O, tetrafluoroethylene and high-molecular viscous compounds – perfluoropolyethylene oxides, their average molecular mass is 104-105. Their structure is as follows:
- (CF2CF2O)a- (CF2O)b- (CF2CF2–OO)c – (CF2–OO)d-
At tetrafluoroethylene oxidation the total quantum yield is higher than at hexafluoropropylene oxidation, but it much depends on reaction’s conditions.
During the temperature drop from - 60oC up to +20oC the peroxides' content decreases.
Fluoroolefines photoxidation mechanism is a complicated process, in which three different types of radicals participate: they are perfluoroalkyl (R*F ), perfluoroalkoxil (RFO*) and perfluoroperoxide (RFOO*).
Here are most important elemental reactions according to works’ data (1,2):
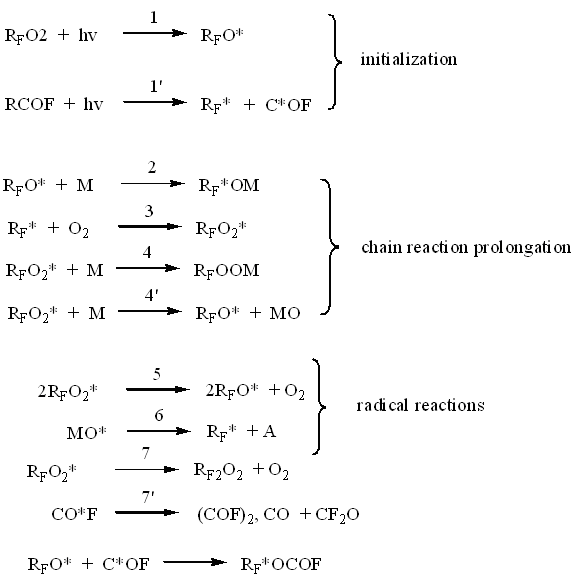
where: M=C2F4 or C3F6; RF =CF3 ,M
A= CF2O, CF3COF
The fluorolefines oxygen photooxidation reactions’ mechanism can be described by the following stages.
A) Initiation Reaction
The reaction is initiated by UV-light of medium and low pressure lamps which wave length is less than 320nm. Admixtures free fluorolefines and oxidation products don't absorb light which wave length is more than 200 nm, in consequence of what the reaction breakdown time is always short, when small amounts of fluorolefine transform into compounds absorbing light such as– CF3COF or peroxides. At peroxides' photolysis O-O link opens / is destroyed, at acylfluorides' photolysis C-C link opens. At that free radicals are formed, initiating chain reaction between olefins and oxygen.
B) Chain Carrying on Reaction
Reactions 2,3,4 are stages of chain growth. Out of them reaction 4 may take place in most cases, though other ways are possible too.
C) Radicals' Reactions
It is known, that perfluoro-alkoxyradicals tend to dissociate according to C-C link even at low temperature (4). Therefore the reactions of 5,6 radicals' dissociation take a biggest part at stage of chain propagation. The structure of final polyether is determined by competition of 2-6 reactions.
D) Chain Termination Reactions
As is shown (3) one of possible chain termination reactions is a reaction of two alkoxyradicals 7 recombination. The bigger part of radicals will recombine by reaction 5, forming two radicals, participating in chain propagation.
As we have mentioned before, the main drawbacks of fluorolefines photochemical oxidation are:
- low process productivity
- Complicated apparatus mounting (sealing of quartz lamps)
- low efficiency of UV-radiation source (93% are IR radiation of heat) etc.
Taking this into account the most progressive is the method of radical fluorolefines and oxygen co-polymerization when using chemical compounds able to homolytically dissociate at low temperatures – for example elemental fluorine.
Using NMR F19 method we determined the structure of perfluoropolyethers on the base of hexafluoropropylene:
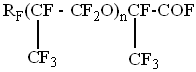 , where RF = CF3, (CF3)2CF-
, where RF = CF3, (CF3)2CF-
The particular feature of it is that in the structure of the compounds virtually there are no fluoroformate and difluoromethyleneoxide groups, which are imperative at photochemical oxidation.
At using of chemical initiation the hexafluoropropylene and oxygen co-polymerization reactions occur according to the following scheme:
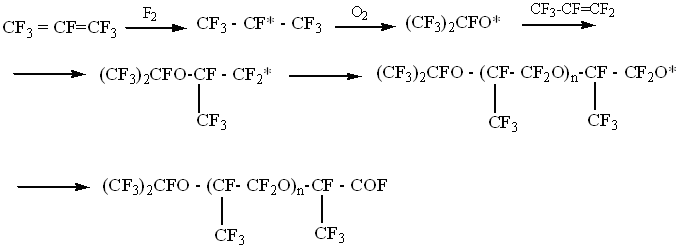
The characteristic property of chemical initiation of hexafluoropropylene and oxygen co-polymerization process is the absence of reaction by-products'inhibitory action (COF2, CO, CO2, CF3COF etc.)
Perfluoropropyleneoxides are reaction products, they are colorless viscous liquids ( =100-400 and more) with density 1,83-1,92 g/cm3and molecular mass 800-10000. Molecular-weight dispending of perfluoropolyethers at synthesis temperature changing from -30 to -50 oC is shifting to high-molecular compounds.
=100-400 and more) with density 1,83-1,92 g/cm3and molecular mass 800-10000. Molecular-weight dispending of perfluoropolyethers at synthesis temperature changing from -30 to -50 oC is shifting to high-molecular compounds.
Perfluoropolyether acids fluoroanhydrides are functional perfluoropolyethers, obtained at chemical initiation using fluorine,
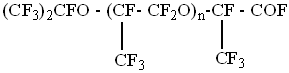
which in the open air slowly hydrolyze forming perfluoropolyether acids in hydrate form (jelly-like mass) .
Fluoroanhydrides are rather reactive compounds and they are used for synthesis of different useful compounds.
References
1. Sianesi D., Am. Chem. Soc., Polymer-Preprints, 1971, v. 12, #1, p. 411
2. Sianesi D., Pasetti A. at all, III Europian Symposium on Fluorine Chemistry, Aix-Eu-Provens, Sept. 1970
3. Hovard I.A., Ingold K., Can. J. Chem., 1965, v. 43, 2729
Fluorine Notes, 2005, 38, 1-2
