Fluorine Notes, 2005, 38, 3-4
Interaction of perfluoro-2-methylpent-2-ene and potassium ethylxantogenate
G.G. Furin
Novosibirsk Institute of Organic Chemistry named after N.N. Vorojtsov of Siberian Division of Russian Academy of Science
fax: +7-3832-344752, e-mail: furin@nioch.nsc.ru
Heterocyclic compounds, containing perfluoroalkyl groups, attract attention of researchers because of biological activity increase [1-3]. However, the majority of such compounds contain oxygen and nitrogen atoms in the cycle. At the same time heterocycles with one or two sulphur atoms in the cyclic system attract a particular attention. Using of heterocyclic compounds with perfluoralkyl groups of perfluorolefines and their derivatives, which have unsaturated functional group at multiply bond, for synthesis allowed to greatly widen the range of researches of such compounds for their biological activity and extending fields of their practical application [4,5].
Nucleophilic reactions of perfluorolefines with binucleophilic reagents (a-b-c triads) are important, here a and c are C,N; N,N; O,N; S,N; S,S atoms and others [6]. The key moment of the present reactions is the generation of new multiply bond during interaction of perfluorolefine and binucleophilic agent, the cyclization according this new bond forms heterocyclic system.
In this work the problem of studying of perfluor-2-methyl-pent-2-en (I) and potassium ethylxantogenate (II) reactions is being solved in terms of development of this methodology of heterocyclic compounds with perfluoroalkyl groups synthesis. The presence of triad S=C-S in this reagent allowed to expect the formation of heterocyclic compounds. The work [7] showed this, where it is shown that sodium N,N-diethyldithiocarbamate (III) together with hexafluoropropylene and perfluoroisobuthylene in dimethylacetamide produces five-membered heterocycle - derivatives of 1,3-dithiolane. However in case of such reaction of reagent (III) with internal perfluoroolefine (perfluor-2-methylpent-2-en) heterocycle compound doesn't form, but unexpectedly we get S-[heptafluoro-4-trifluoromethylpent-2-en-3-yl]-N,N-diethyldithiocarbamate, that shows us an important role of perfluorolefine used.
We have stated, that at the reaction of olefine (I) with potassium ethylxantogenate in DMF at 45 oC the fluorine atom substitution product was formed at internal multiply bond (IV) and also the compound already known (V). Further boiling of compound (IV) in DMF in the presence of potash (K2CO3) results in obtaining of (VI) and (VII) compounds mixture.
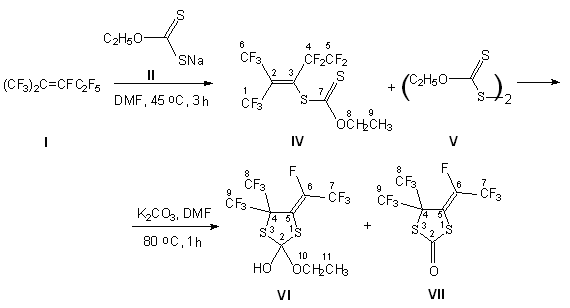
The position of F and CF3 substitutes at C=C multiply bond in (VI) and (VII) compounds must be determined by steric factors. In the presence of two CF3groups at C4 atom the E-isomer must be less tense, its fluorine atom is turned to them. Before such disposition was confirmed by X-ray structure analysis for few 2-substituted derivatives of 4,5-dihydro-1,3-thiazole, possessing the same fragment [8]. Besides that, in NMR 19F spectra the signal of this fluorine atom has a quartet septets structure due to interaction with fluorine atoms in CF3 groups at C4 atom [1,3] of dithiolane ring (JFF 20 Hz) and with fluorine atoms of CF3 group, being at multiply bond (JFF 10 Hz).
The formation of compound (VII) must be going according to the following scheme:

The compound (VII) must be a result either of (VI) compound thermolysis or eliminationof ethyl cation from intermediate salt (VIII).
The composition and structure of synthesized compounds are confirmed by elemental analysis data, NMR 1H 19F (they are interpreted taking into account the information we have for such type compounds), IR-spectra and mass spectrometry. The examination of NMR 1H and 19F spectra shows, that they are characterized by the same regularities for nucleus screening and by analogous constants of spin-spin coupling, which were observed in such objects before [9,10].
Thus, at certain conditions the interaction of perfluoro-2-methylpent-2-en and ethylxantogenate potassium salt results in construction of five-membered heterocycle due to participation in the processes of multiply bond intramolecular cyclization.
Experiments
NMR 1H and 19F spectra are obtained at the Bruker WP 200SY spectrometer (200, 188 MHz respectively), the values of chemical shifts are given in regard with internal standards - TMS, C6F6 . IR-spectra (5 % in CCl4) were recorded at the Fourier- spectrometer IFS66. Mass-spectra were recorded at chromato-mass-spectrometer (Hewlett Packard G 1800A GCD system, electron energy 70 eV). We used 30 m column, its diameter 0.25mm, coated inside with 5%-diphenyl-(95%)-dimethylsiloxane (HP-5, 0.25 micrometer thickness), gas-carrier - helium, 1 ml/min, evaporator temperature was 280 oC. Column temperature was being raised from 50 oC (held for 2 min) at the rate of 10 degree /min up to 280 oC (held for 5 min). All reactions were controlled using NMR 19F method. The analysis of reaction mixtures was held at chromotograph LHM 72 (15 % SE, SKTF-803 : QF-1, chromosorb W, 4000 mm column, diameter - 4 mm).
Ethyldioxisulfocarbonate (V) Melting point. 28-29 oC, FC(S)NEt2 (boiling point 53-54 oC (16 mm Hg).
Compound 1 (6 g) was added at intensive stirring to a suspension of ethylxantogenate potassium salt ( 4g ) in anhydrous DMF (40 ml) at room temperature. The reaction mixture was stirred for 3 hours at 45oC, cooled, poured into water, extracted by CH2Cl2 and dried over CaCl2. After vacuum distillation of the solvent the residue was distilled.
O-ethyl Dithiocarboxylic acid Ester
S-(perfluoro(2-methyl-1-ethyl)-propenyl ether (IV), yield 4.2 g (62 %),
product of yellow color., Boiling point 60-61 oC(3 mm Hg.). NMR 1H (CDCl3),
![]() H,
ppm : 4.62 d (2H, CH2 ,JHH 7.8
Hz ), 1.40 t ( J = 7.8 Hz); NMR 19F (CDCl3).
H,
ppm : 4.62 d (2H, CH2 ,JHH 7.8
Hz ), 1.40 t ( J = 7.8 Hz); NMR 19F (CDCl3).
![]() F,
ppm :
105.5 qt (F6, 10; 20 Hz), 103.1 s ( F1), 84.5 q (3F, F5, J = 10
Hz), 58.3 q (2F, F4, J
= 20 Hz). IR-spectra cm-1 : 2920 (C-H), 1670 (C=C), 1550 (C-O),
1150-1250 (C-F), 1030, 1060 (C-O). Found, % :
C, 26.77; 27.03; H, 1.26; 1.38; F, 53.06; 52.77; S, 126.00; 16.30. C9H5F11OS2.
Calculated, % : C,
26.87; H, 1.24; F, 51.99; S, 15.92.
F,
ppm :
105.5 qt (F6, 10; 20 Hz), 103.1 s ( F1), 84.5 q (3F, F5, J = 10
Hz), 58.3 q (2F, F4, J
= 20 Hz). IR-spectra cm-1 : 2920 (C-H), 1670 (C=C), 1550 (C-O),
1150-1250 (C-F), 1030, 1060 (C-O). Found, % :
C, 26.77; 27.03; H, 1.26; 1.38; F, 53.06; 52.77; S, 126.00; 16.30. C9H5F11OS2.
Calculated, % : C,
26.87; H, 1.24; F, 51.99; S, 15.92.
2. The mixture of 3 g of compound (IV) and 1.5 g of K2CO3 in 20 ml of DMF during stirring was warmed up at 80 oC for 1 hour, poured into the water and extracted by ether. Ether extract was dried using CaCl2, concentrated in the vacuum of water-jet pump. The residue was forwarded onto column with silica gel and in the CH2Cl2 acetone (5:1) system we isolate the reaction product.
First spot:
(E)-4,4-Di(trifluoromethyl)-5-tetrafluoroethylidene-[1,3]-dithiolan-2-on
(VII) , yield 0.5 g (19 %), boiling
point 80-85oC (7 mm Hg). Spectra NMR 19F (CDCl3).
![]() F,
ppm :
97.1 d (F7,8, 10 Hz), 94.9 d ( F9 , 20 Hz), 47.8 hept. triplet (F6,
J = 20; 10 Hz). IR-spectra cm-1 : 1730
(C=O), 1670 (C=C), 1570 (C-O),
1130-1150 (C-F), 1030, 1060 (C-O). Found, %: C,
23.84; 23.69; F, 53.98; 53.71; S, 18.20; 18.00. C7F10OS2.
Calculated, % : C, 23.73; F, 53.67; S, 18.08/
F,
ppm :
97.1 d (F7,8, 10 Hz), 94.9 d ( F9 , 20 Hz), 47.8 hept. triplet (F6,
J = 20; 10 Hz). IR-spectra cm-1 : 1730
(C=O), 1670 (C=C), 1570 (C-O),
1130-1150 (C-F), 1030, 1060 (C-O). Found, %: C,
23.84; 23.69; F, 53.98; 53.71; S, 18.20; 18.00. C7F10OS2.
Calculated, % : C, 23.73; F, 53.67; S, 18.08/
Second spot :
(E)-2-Etoxy-4,4-di(trifluoromethyl)-5-tetrafluoroethylidene-[1,3]-dithiolan-1-ol (VI), yield 1
g (33.5 %), boiling point 97-98 oC(7 mm Hg). Spectra NMR 1H (CDCl3),
![]() H,
ppm : 4.62 q (2H, CH2),
1.39 t ( J = 5.8 Hz); Spectra NMR
19F (CDCl3).
H,
ppm : 4.62 q (2H, CH2),
1.39 t ( J = 5.8 Hz); Spectra NMR
19F (CDCl3).
![]() F, ppm:
105.5 qt (F8, 10; 20 Hz), 104.4 s ( F9), 97.1 d (F7,
J = 10 Hz), 47.8 hept. triplet (F6,
J = 10; 8 Hz). IR-spectra, cm-1 : 2920 (C-H), 1670 (C=C), 1570
(C-O), 1490 (C-O), 1130-1250 (C-F), 1030, 1060 (C-O).
Found, % : C, 26.84; 26.72; H, 1.64; 1.47; F, 48.00; 47.81. C9H6F10O2S2. Calculated, % : C, 27.00; H, 1.50; F,
47.50.
F, ppm:
105.5 qt (F8, 10; 20 Hz), 104.4 s ( F9), 97.1 d (F7,
J = 10 Hz), 47.8 hept. triplet (F6,
J = 10; 8 Hz). IR-spectra, cm-1 : 2920 (C-H), 1670 (C=C), 1570
(C-O), 1490 (C-O), 1130-1250 (C-F), 1030, 1060 (C-O).
Found, % : C, 26.84; 26.72; H, 1.64; 1.47; F, 48.00; 47.81. C9H6F10O2S2. Calculated, % : C, 27.00; H, 1.50; F,
47.50.
The work is accomplished in the framework of the RF Ministry of Education program named "Universities of Russia" (grant UR.05.01.023).
References
[1] G.G. Furin / Fluorine containing heterocyclic compounds. Synthesis and application. Novosibirsk: Science 2001. 304 p.
[2] K. Burger, U. Wucherpfenning, E. Brunner // Adv. Heterocycl. Chem., 1994. Vol. 60. P. 1-64.
[3] R.D. Chambers, C.R. Sargent // Adv. Heterocycl. Chem. 1981. Vol. 28. P. 1-71.
[4] R. Filler, Y. Kobayashi / Biomedical aspects of fluorine chemistry. Tokyo; N.Y. : Kodansha Elsevier Biomed. Press. 1982; Studies in organic chemistry. 48. Organofluorine compounds in medicinal chemistry and biomedical applications. / Eds. R. Filler, Y. Kobayashi, L.M. Yagupolskii. Amsterdam : Elsevier Sci. Publ. 1993. 386 p.
[5] J.T. Welch, S. Eswarakrishman / Fluorine in bioorganic chemistry. N.Y. : Wiley. 1991.
[6] G.G. Furin, E. L. Zhuzhgov // Russian Chemistry of Heterocyclic Compounds. 2002. # 2., P.147-171.
[7] T. Kitazume, S. Sasaki S., N. Ishikawa // J. Fluorine Chem., 1978. Vol. 12. P. 193-202/
[8] G.G. Furin, A.V. Rogosa, I.U. Bagrianskaya, U.V. Gatilov // Russian Journal of Organic Chemistry. 1997. Vol. 33. Issue 5. P. 787-795.
[9] G.G. Furin, L.S. Pressman, I.A. Salmanov, E. L. Zhuzhgov // Russian Journal of General Chemistry, Vol.. 69. Issue 9. P. 1499-1503.
[10] A.V. Rogosa, G.G. Furin // Russian Journal of General Chemistry, 1999. Vol.. 69. Issue 9. P. 1491-1498.
Information about the Author
Georgy G. Furin, Doctor of Chemical Sciences, Research officer in-chief, Head of the Fluorine-containing Elementoorganic Compounds Group of Novosibirsk Organic Chemistry Institute SD RAS.
Address : 630090, Novosibirsk-90, Academician Lavrentiev av., 9
Novosibirsk Organic Chemistry Institute; Siberian Department of Russian Academy of Sciences.
Tel.: work +7(3832) 344747,
Home +7(3832)
355389
Fax : +7(3832) 344752
E-mail : furin@nioch.nsc.ru
Fluorine Notes, 2005, 38, 3-4
