Fluorine Notes, 2004, 37, 1-2
Perfluorinated Carboxylic Acids. Synthesis and Application.
Novosibirsk Organic Chemistry Institute of named after N.N. Vorojtsov Of RAS Siberian Department
Ac. Lavrentiev avenue, 9. Novosibirsk, Russia, 630090
E-mail : furin@nioch.nsc.ru
Dedicated to Professor Hermann-Josef Frohn on the Occasion of his 60th Birthday
In this article you will find data regarding perfluorinated carboxylic acids obtaining methods, in particular electrochemical fluorination of hydrocarbon derivatives in anhydrous hydrogen fluoride, perfluoroolefines and telomeric alcohols oxidizing, perfluoralkyliodides and carbon dioxide interaction in the presence of initiators. Some characteristics of perfluorinated carboxylic acids are discussed in this article. Using of these characteristics for creating of new semi-products, used for fluororganic synthesis is discussed here. Practical application examples of both perfluorinated carboxylic acids and semi-products based on them for creating of new generation fluorine materials are given in this article.
Table of content
1. Introduction. The role of fluorine compounds in development and perfection of materials for the new techniques.
2. The Synthesis of Perfluorinated carboxylic acids.
2.1. Electrochemical fluorination of carboxylic acids and some of their derivatives in anhydrous hydrogen fluoride.
2.2. Oxidization processes of linear and cyclic perfluorolefines and other fluorine-containing compounds.
2.2.1. The perfluorolefines reactions with ozone.
2.2.2. Innovations in the oxidization processes of perfluorolefines' double bond up to perfluoroalkancarboxylic acids.
2.2.3. The oxidization of telomeric alcohols and polyfluoroaromatic compounds.
2.2.4. The transformations of perfluoroalkylhalogenides into perfluorocarboxylic acids under the initiators action.
2.2.5. The synthesis of perfluorocarboxylic acids out of other classes compounds.
3. The characteristics of perfluorocarboxylic acids and practical application of fluorine materials obtained on their basis.
3.1. Decarboxylation and decarbonization of perfluorocarboxylic acids.
3.2. Surface-active materials on the base of perfluorocarboxylic acids derivatives.
3.3. Surface-active agents as fire-extinguishing materials.
3.4. Compositions for treatment of articles surfaces, rust-proofing coatings.
Conclusion
References.
3.2. Fluorine-containing Surfactants Based on Perfluorocarboxylic Acids Derivatives.
Fluorine-containing surfactants are ordinary surface active materials of hydrocarbon type, in which hydrogen atoms of carbon chain are partly or totally replaced by fluorine atoms, in which connection they show a number of specific features [134].
At first, their high resistance to chemical reagents that is acids, alkalis etc. should be noted, and their heat resistance should be also noted.
Secondly, surface activity of fluorine-containing surfactants is higher, than the one of ordinary hydrocarbon surfactants. They are extremely effective for decreasing of surface tension (they form films on the surface of water and acids, which prevent isolating of metals and vapours of different compounds out of electrolytes). At absorption on the surface of water fluorine-containing compounds can decrease surface tension of water down to 15 din/cm.
Thirdly, as surfactants of this type are effective at extremely low concentration, we can expect decreasing of used compound amount, that besides the economical benefit gives great advantages in the view of environmental control. The production of a number of fluorine containing surfactants is commercialized.
Basically, synthesis methods of fluorine-containing surfactants are based on turnings of fluorine-containing semi-products into poly-fluorinated carboxylic acids, which derivatives possess surface-active features. Among them 3 groups of processes are marked:
- electrochemical fluorination, when as starting material hydrocarbon analogues are used including telomeric alcohols like H(CF2CF2)nCH2OH (n = 3-7),
- telomerization of unsaturated fluorine-containing compounds, for example, telomerization of commercially produced tetrafluoroethylene at action of UV-light with formation of perfluoroalkyliodides and following action of carbon dioxide on them,
- olygomerization, for example, hexafluoropropylene in the presence of fluoride-ion or fluoroahnydride of perfluorocarboxylic acid in the presence of fluoride-anion.
In these methods the obtained semi-products contain a group at the end of olygomer, possessing high reactivity. As a rule, the reactions of perfluorocarboxylic acids fluoroanhydrides are carried out with nucleophilic reagents, and reactions of unsaturated perfluorinated compounds are carried out with binucleophilic reagents or nucleophilic reagents, having carboxylic groups. As in perfluoroalkyl iodides the iodid atom detaching in the form of iodide-anion is complicated under the influence of perfluoroalkyl radical, the additional reaction with ethylene is carried out and in such a semi- product the mobility of iodide atom is used to build the necessary system.
Determining opportunities of fluorine-containing surfactants as water-oil-repelling the critical wetting surface tension value offered by Zisman [135] is convenient ( c). It turned out, that the surface tension of aqueous solutions of ordinary surfactants can be lowered down to 25-27 din/cm, while the fluorine-containing ones down to 15-20 din/cm.
c). It turned out, that the surface tension of aqueous solutions of ordinary surfactants can be lowered down to 25-27 din/cm, while the fluorine-containing ones down to 15-20 din/cm.
The most effective ability of perfluorocarboxylic acid derivatives is to produce films on the surface of aqueous solutions with carbon chain length equal to 6-8. As fluorocarboxylic chain length is growing the decresing of surface tension is observed and when the chain length is the same it depends on the gegenion type : the essential decreasing of surface tension is observed for compounds, containing H+, NH4+, NH3+C2H4OH as gegenion. In recent years the following amines are used:
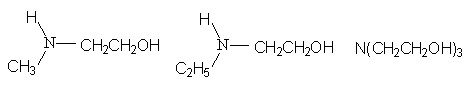
In table 6 the minimal values of perfluorocarboxylic acid salts aqueous solutions surface tension are listed [136].
Table 6.Minimal surface tension values of salts aqueous solutions with the formula CnF2n+1COOH at 25 oC [136].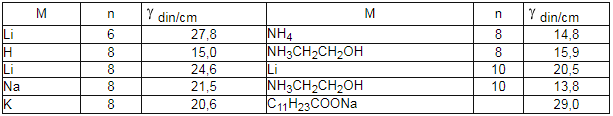
Etherification of perfluorocarboxylic acid like CnF2n+1COOH (n = 6, 7, 8) using polyethyleneglycoles - HO(CH2CH2O)mH (m = 4, 8, 13) in the presence of 0,5 % concentrated sulfuric acid results in formation of CnF2n+1COO(CH2CH2O)mH ethers, which possess properties of surfactants [137]. Aqueous solutions of these ethers form dense foam with the height ranging from 70 mm to 160 mm, which is practically not falling in 5 minutes. The values of surface tension allow to use these ethers for treatment of polyester material, possessing good anti-static properties.
Fluorine-containing surfactants are characterized by high resistance to strong acids and to concentrated alkalis and they effectively lower surface tension in such mediums. In alkali solutions fluorine-containing surfactants in small concentrations lower surface tension down to very low values. The addition of fluorine-containing surfactants to alkali cleaning compositions greatly improves their effectiveness. The compositions of such type are effective for removing coatings from steel plates surface and compounds adsorbed on it.
Fluorine-containing surfactants can be used in the following fields: galvanization, microelectronics, chemical and photographical industry. They can be used to create compositions of surfactants low-energetic sorbtion layers on the surface of solid bodies, that allows to low the constant of friction and wear of contacting surfaces, to increase durability and their working potential.
Salts can be used as foam makers for fire-fighting instead of foam-maker "light water" of 3M company (USA). The formation of foam on the surface of hydrocarbons impedes the access of air oxygen.
Fluorine-containing surfactants inhibit the metal corrosion under the action of acids. They are effective both at introduction into acidic medium and at covering the surface of metal, contacting acid, with thin protective film. As at electrochemical chromium-plating baths, containing sulfuric acid and possessing strong oxidizing action of chrome compounds, the process is carried out at high current densities.
In these conditions the ordinary organic surfactants decompose. Fluorine-containing surfactants don't decompose in conditions of electrochemical fluorination and decrease the surface tension of bath, calling for formation of a steady layer of foam on its surface and thus preventing from isolation of poisoned smoke. They can also decrease the rate of chromium-plating and lower the corrosion of the object being chromium-plated. Fluorine-containing surfactants are also applied at electrolytical copper plating and nickel-plating.
Attention should be paid to the fact, that fluorine-containing surfactants do not only exceed their hydrocarbon analogues by foam- making ability, but also produce thinner and more stable foam, heat and chemical reagents resistant.
The foam-making ability of fluorine-containing surfactants are not subject to the influence of electrolytes. They are also used to apply protective films on the surface of metals. Such films are water and oil resistant. Copper and brass processed using this method stay shiny and do not corrode even in the atmosphere containing hydrogen sulfide or sulphur dioxide. They possess oil-repellent properties of solid surface. At interaction of fluorine-containing surfactants into emulsions of synthetic resin an extremely thin homogeneous foam, possessing high stability and allowing to obtain molded items of high porouness, for example for polyurethane is formed.
Surfactants (produced) on the base of poly-fluorinated carboxylic acids with the number of carbon atoms more than 6 possess aerosol inhibiting effect at electrolysis of different metals and do not negatively influence on the quality of cathode metal and on current yield.
3.3 Surfactants - Fire Extinguishants.
With the advent of new materials made of synthetic organic compounds the problems of their inflammation protection arose. The ordinary fire extinguishants are not effective for them. Moreover the worked out fire extinguishants based on perfluoroalkylchlorides and perfluoroalkylbromides, appeared to be ozone unsafe and their production is stopped. Foam is widely used for fire extinguishing at industrials plants, oil storages, transport etc. Foam is a dispersion, consisting of gas bubbles, surrounded by liquid films, and it is characterized by thermodynamic instability regarding the aggregate one.
Aerosolson the base of organic compounds also possess a number of disadvantages. Fire extinguishants on the base of fluorine-containing compounds have more potential. They can be divided into several groups. The main among them are:
1. Extinguishants, consisting of ozone safe chladones (freons),
2. Foam former, intended to obtain air-mechanical foam for fire extinguishing.
In spite their high cost the compounds on the base of fluorine-containing compounds have rather fast proved their effectiveness and they are used both for common fire extinguishing, fire extinguishing of reservoirs with petroleum and other organic solvents and end use, in particular, of cars, movable installations and places with mass people stay.
Earlier, freons, mainly bromine-containing ones, had found their application in different field of techniques. However, they are ozone destructive and according to Montreal Protocol were prohibited for operation. Russia follows this protocol and production of such chladones (chladones 13B1, 12B1, 114B2 and other) now is stopped in Russia.
Compounds close to them by physical properties and reproducing their high operational characteristics are considered as an alternative for bromo-chladones.
On the basis of defined in laboratory conditions fire extinguishing concentrations of ozone safe compounds and opportunity of their production as most promising ones for application chladones 125 and 227ea were chosen. At that the most preferred is chladone 125 [138]. The action of present chladones lies in formation of aerosol cloud, which covers the extinguished object, that prevents air oxygen to pass into combustion zone or blocks its inflow. Because of this the combustion is ended.
The practical interest in wide group of different purposes' fluorine-containing surfactants is constantly increasing, that causes search for optimal, available for commercial production compounds. Fluorine-containing surfactants of cationic, anionic and nonionic types with necessary physical - chemical and colloid-chemical properties are created.
Foams made out of fluoroorganic surfactants are effective, because solution, isolating from foam, gives film on the surface of combustible liquid, this film prevents foam from destruction. They are chemically and thermally stable, biologically resistant and their surface stability is high. High fire extinguishing capability of film-forming compounds on the base of fluorine compounds allows to feed foam under to the layer of combustible and also spraying fromover it, that facilitates the fire extinguishing process. At layer-by-layer method of fire-extinguishing the coming to surface foam can bypass constructions sunken in reservoir and spread all over the surface later. After fire extinguishing and ending of feeding all over the combustible summary surface the stable layer with the thickness up to 5 cm is formed, which prevents it from re-inflammation. Suchfoam formers on the base of fluorine-containing surfactants found their application for fire extinguishing of both polar and non-polar liquids (benzine, acetone, kerosene etc.) and also for A and B class fire extinguishing.
High reliability of fire-extinguishing, particularly the high resistance to re-inflammation of laying under the foam layer liquid already extinguished is also referred to the advantages of such materials. Foam formers on the fluororganic compounds are hard- to -inflame liquids, and their work solutions are fire-safe and explosion-proof. In industry foar formers are produced by solution of fluoroorganic concentrate in water. Mainly, the following fluorine materials: PO-6 FP, Foretol, "Universal" find their application. The intensity of frother feed at fire extinguishing of standard fuel (n-hexane) using foam of medium multiplicity, kg/m2s is : 0.05, 0.08, 0.08. Work concentration % vol. 6, 10, 10 respectively. They are homogeneous coloured liquids without sediment and fibering.
Technical requirements for complete product (work solution)
Universal | Foretol | |
| Foam multiplicity | 6 | 6 |
| Foam stability at/on the base of ethyl alcohol, min, no more than | 10 | 10 |
| Period of ethyl alcohol extinguishing, sec, no more than | 50 | 50 |
Purpose of use/designation:
- PO-6 FP- for fire extinguishing of hydrocarbons using air-mechanical foam with fresh water
- Foretol - for fire extinguishing of burning polar liquids (alcohols, organic acids etc/) using air-mechanical foam with fresh water
Action of fluorine-containing Foam former lies in the fact, that if foam gets over burning liquid, then solution, isolating form it, forms a film on the surface of combustible liquid, which prevents combustible vapours from entering into zone of burning and protects foam from destruction, as a result of that a rather high fire extingyuishing effect is reached.
In Russia the production of such fire extinguishing facilities is organized as test version (layer-by-layer frother PO-6 FP (TU 241279-130-05807960-97)) at JSV Halogen (Perm city). Foam former named “Easy water” of 3M company (USA) is analogue of PO-6 FP. Foam former PO-6 FP is a biologically degradable product, the rate of biodegradation > 80%. Application limitation – can't be used for fires of B2, C, D classes.
3.4. Compositions for Surface Treatment of Products, Antirust Coatings.
The need for high-temperature lubricants, which at treatment of moving components increase the operation life of mechanisms, is great, because their friction coefficient is low. Along with that they can be used as lubricants of both rolling mills for steel and for other metals. Other field of application is for oil equipment and oil pipelines, because such lubricants are not washed out by organic compounds compare to mineral oils.
A group of chemicals for treatment of waterproof cotton fabric with high consumer properties was created on the base of perfluorinated carboxylic acids [139]. Fluorocarboxylic chemical AFS (Hechst, Germany) gives the best water-repellent ability to fabric. Fabric trimming using this chemical results in improving of stability of shape, moisture resistance, fabric strengh, light resistance etc.
Fluorinated surfactants found their application in technics as modifiers of solid surfaces for giving antiadhesive, antofriction, antiscruff, antiwear, corrosion preventing properties to them. [140].
Fluorine-silicon organic compounds on the base of perfluorocarboxylic acids' amides are used for treatment of monuments, they create a protective layer on their surface, blocking moisture, acid admixtures and industrial pollution penetration into pores of buhr/limestone. [141]. These materials are obtained according to the following scheme:
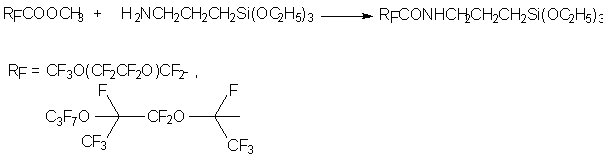
The material, we had discussed in this review, shows a growing researchers' interest to development of new introduction methods of perfluorinated fragments into molecules of organic compounds and methods of ordinary substituents' transforming into complicated functional groups. Noticeable success, reached in creation of new perfluoroalkylating agents show, that in a few cases they can be an alternative for classical and well-known reagents.
Thus, perfluoroalkyliodides are widely used to carry out perfluoroalkylating reactions in the presence of initiators and catalysts. Such processes as radical perfluoroalkylating, formation of perfluoroalkylsulfine and perfluorocarboxylic acids, poly-fluorinated alhydes, electrochemical synthesis of perfluoroalkyl derivatives have obvious advantages, they are rather simple and can be commercially realized. We can hope, that later new reactions and transformations resulting in fluorine-containg compounds' formation, would be found.
In this review there was made an attempt to demonstrate new approaches and synthetic opportunities of new reagents, to show tendencies and main directions of researches in the field of perfluoroorganic compounds, containg different molecular skeletons and functional groups. The discussion over material appeared to be very useful, because it often allowed to see the problem from the untraditional for chemists point of view, to find some original solutions, based on use of new approaches to generation of perfluoroalkyl radicals, their involving into reactions with different compounds. As reaction of perfluoroalkyliodides with alkenes is one of the most important fundamental reactions not only in fluororganic compounds' chemistry but also in organic chemistry in general, it's obvious that more delicate work is necessary to solve these puzzling problems.
There is no doubt, that implementation of discussed above ideas and processes, passing according to the scheme of one-electrone transfer is of interest not only of chemists-fluororganics, but also of specialists in the field of organic synthesis, of scientists developing fundamentally new influiencing methods on organic compounds molecules with the purpose of creating new technologies. At that you can see, that in a number of cases perfluoroorganic compounds are convenient and sometimes unique models both for creation of new effective reagents and for setting and solving a number of fundamental problems of theoretical organic chemistry. Thereby the role of perfluorine-containing organic materials is increasing non-stop, though only a small number of perfluoroalkylating agents, which could be commercially used, is known so far. However, soon you can expect a great progress in this field. Thus, the effective working out of methods transforming organic compounds into valueable fluorine-containing products will promote the progress of organic synthesis as a whole.
References
1. Organofluorine chemistry. Principles and commercial Applications. / Banks R.E., Smart B.E., Tatlow J.C. (Eds). Plenum Press : New York. 1994.
2. Weinberg N.L. // in Techniques of chemistry/ Weinberg N.L. (Ed.). Wiley : New York. 1995. Vol. 5. P. 1-82.
3. Drakesmith F.G. // Topics in Current Chemistry. Springer : Berlin. 1997. Vol. 193. P. 197-242.
4. Fluorine Chemistry : A Comprehensive Treatment. Ed. M. Howe-Grant. Encyclopedia Preprint Series. Wiley. 1995, pp. 304-318.
5. Shibuta D., Omori K., Takenuki S. / Pat. Japan 62-69186 (1987); Chem. Abstr. 1988. Vol. 108. 13081a.
6. Prokop H.W., Zhou H.-J., Xu S.-Q., Wu C.-H., Chuey S.R., Liu C.C. // J. Fluorine Chem. 1989. Vol. 43. N 2. P. 277-290.
7. Comninellis C., Javet P., Plattner E. // J. AppI. Electrochem. 1974. Vol. 4. P. 289.
8. Drakesmith F.G., Hughes D.A. // J. Fluorine Chem. 1986. Vol. 32. N 1. P. 103-134.
9. Lines D., Sutcliffe H. // J. Fluorine Chem. 1981. Vol. 17. P. 423.
10. Chambers R.D., Fuss R.W., Jones M., Sartori P., Swales A.P., Herkelmann R. // J. Fluorine Chem. 1990. Vol. 49. P. 409-419.
11. Brit. Pat. 1077301 (1967); Abe T., Hayashi E. / Pat. Japan 61-260047 (1986); Chem. Abstr. 1987. Vol. 107. 105032.
12. Blochl W. / Pat. 1436269 Fr. (1966).
13. Scholberg H.M., Bryce H.G. / Pat. USA 2717871 (1955).
14. Brice T.J., Pearlson W.H., Schoeberg H.M. / Pat. 2713593 US (1955).
15. Gramstad T., Haszeldine R.N. // J. Chem. Soc. 1956. P. 173; 1957. P. 2640.
16. Alekseev B.P., Bil`dinov K.N., Galkina N.I., Pozdeeva G.A., Rastorgueva N.M., Serebrov P.V., Shcherbakova M.S. // Pat. 503872 U.S.S.R. (1976); C.A. 1976. Vol. 85. 46634t.
17. Wasser D.J., Johnson P.S., Klink F.W., Kucera F., Liu C.C. // J. Fluorine Chem. 1987. Vol. 35. N 3. P. 557-569.
18. Young J.A., Dresdner R.D. // J. Am. Chem. Soc. 1958. Vol. 80. P. 1889.
19. Kauck E.A., Simons J.H. / Pat. USA 2644823 (1953).
20. Rudge A.J. Industrial Electrochemical Process. (Ed.) A.T. Kuhn. Elsevier : Amsterdam. 1971. P. 77.
21. Ignat`ev N.V., Welz-Biermann U., Heider U., Kucheryna A., Ahsen S., Habel W., Sartori P., Willner H. // J. Fluorine Chem., 2003. Vol. 124. P. 21-37.
22. Abe T., Kodaira K., Baba H., Nagase S. // J. Fluorine Chem. 1978. Vol. 12. P. 1-25.
23. Abe T., Baba H., Hayashi E., Nagase S. // J. Fluorine Chem. 1983. Vol. 23. N 2. P. 123-146.
24. Abe T., Hayashi E., Baba H., Nagase S. // J. Fluorine Chem. 1984. Vol. 25. P. 419.
25. Abe T., Nagase S. / Pat. Japan 80-18539 (1980); Chem. Abstr.1980. Vol. 93. P. 8020.
26. Hansen J.C., Moore G.G.I., Polson S.D., Savu P.M., Spern R.M. / Pat. 6110976 US (2000)
27. Cao W., Ge W., Huang W. // Org. Chem. 1987. N 2. P. 133-137
28. Patent 63-208572 Japan (1988)
29. Hayashi E., Abe T., Baba, S. Nagase // Chem.Express. 1988. Vol. 3. P. 191-194.
30. Abe T., Hayashi E., Fukaya H., Baba H. // J. Fluorine Chem. 1990. Vol. 50. P. 173-196.
31. Berenblit V.V., Byzov B.A., Grachev V.I., Dolgopol'skii I.M., Dolnakov Yu.P. // Zh. Prikl. Khim. 1975. Vol. 48. N 3. P. 709-711; Chem. Abstr. 1975. Vol. 82. 161753.
32. Abe T., Hayashi E. // Pat. 6470450 Japan (1989); Russ. Chem. Abstr., 1990, 12N 329P; Pat. 6470449 Japan (1989); Russ. Chem. Abstr. 1990. 12 N 326P.
33. Abe T. / Pat. 62-22756 Japan (1987)
34. Abe T., Hayashi K./ Pat. 4985556 US (1991)
35. Abe T. / Pat. Japan 63-22546 (1988); Chem. Abstr. 1989. Vol. 110. 38609.
36. Abe T. // Pat. Japan 01-70444 (1989); Chem. Abstr. 1989. Vol. 111. 133633r.
37. Abe T., Hayashi E. / Pat. 61-260047 Japan (1986); Chem. Abstr. 1987. Vol. 107. 105032t.
38. Abe T., Hayashi E. // Chem.Lett. 1988. P. 1887.
39. Abe T., Baba H., Okuhara K., Fukaya H. // J. Fluorine Chem. 2001. Vol. 111. N 2. P. 115-128.
40. Hayashi E., Fukaya H., Abe T., Omori K. // Chem. Lett. 1990. N 5. P. 737-738
41. Abe T., Baba H., Soloshonok I. // J. Fluorine Chem., 2001. Vol. 108. P. 215-228.
42. Abe T., Hayashi E., Baba H., Fukaya H. // J. Fluorine Chem. 1990. Vol. 48. P. 257-279.
43. Abe T., Hayashi E. // Pat. Japan 01-259188 (1989); Chem. Abstr. 1990. Vol. 112. 242200.
44. Abe T., Hayashi E. / Pat Japan 01-70449 (1989); Chem. Abstr. 1989. Vol. 111. 133649.
45. Abe T., Fukaya H., Ono T., Hayashi E., Soloshonok I., Okuhara K. // J. Fluorine Chem. 1998. Vol. 87. N 3. P. 193-202.
46. Abe T., Hayashi E., Fukaya H., Hayakawa Y., Baba H., Ishikawa S., Asahino K. // J. Fluorine Chem. 1992. Vol. 57. N 1. P. 101-111.
47. Abe T., Fukaya H., Hayashi E., Hayakawa Y., Nishida M., Baba H. // J. Fluorine Chem. 1994. Vol. 66. N 1. P. 193.
48. Abe T. / Pat. Japan 62-22772 (1987)
49. Abe T. / Pat. 62-22773 Japan (1987)
50. Berenblit V.V., Byzov B.A., Dolgopol`skii I.M., Dolnakov Yu.P. // Zh. Prikl. Khim. 1974. Vol. 47. P. 2433-2435.
51. Maksimov B.N., Kosareva L.N., Ryabinin N.A. // Pat. 2107751 Russia (1993). BI N 9. P. 146.
52. Hintzer K., Qiu Z.-M., Moore G.G.I., Schwertfeger W., Schulz J.F., Worm A.T., Gross C.L. // Pat. 6482979 U.S. (2002)
53. Mashino M., Ninomiya Y., Kawasaki M., Wallington T.J., Hurley M.D. // J. Phys. Chem. A., 2000. Vol. 104. N 31. P. 7255-7260; Chem. Abstr., 2000. Vol. 133. 107684.
54. Moiseeva N.I., Gekhman A.E., Rumyantsev E.S., Klimanov V.I., Moiseev I.I. // Izv. Akad. Nauk SSSR. Ser. Khim., 1989. N 7. P. 1706; Chem. Abstr., 1990. Vol. 112. 76130.
55. Szlavik Z., Tarkanyi G., Skribanek Z., Vass E., Rabai J. // Org. Lett., 2001. Vol. 3. N 15. P. 2365-2366; Chem. Abstr., 2001. Vol. 135. 210736b.
56. Odinokov V.N., Akhmetova V.R., Savchenko R.G., Bazunova M.V., Fatyechov A.A., Zapevalov A.Ya. // Izv. Akad. Nauk SSSR, Ser. Khim., 1997. N 6. P. 1239-1241.
57. Moiseeva N.I., Gekhman A.E., Rumyantsev E.S., Moiseev I.I. // J. Fluorine Chem., 1989. Vol. 45. P. 136.
58. Odinokov V.N., Akhmetova V.R., Savchenko R.G., Bazunova M.V., Paramonov E.A., Khalilov L.M. // Izv. Akad. Nauk SSSR, Ser. Khim., 2000. N 6. P. 1109-1111.
59. Muidinov M.R. // Rossiiskoe Khim. Zh., 2002. Vol. 46. N 3. P. 72-74; Chem. Abstr. 2003. Vol. 138. 56921.
60. Caminade A.M., LeBlanc M., Khatib M. // Tetrahedron Lett., 1985. Vol. 26. P. 2889.
61. Kiryukhin D.P., Barkalov I.M., Ismoilov I.L. // Khimicheskaya Fizika. 2003. Vol. 22. N 2. P. 123-128; Chem. Abstr. 2003. Vol. 139. 133862.
62. Odinokov V.N., Akhmetova V.R., Bazunova M.V., Paramonov E.A., Khalilov L.M. // Mendeleev Commun., 1989. P. 83-128.
63. Denmark S.E., Wu Z., Crudden C.M., Matsuhashi H. // J. Org. Chem., 1997. Vol. 62. N 24. P. 8288-8289.
64. Battais A., Boutevin B., Pitrasanta Y., Sierra P. // J. Fluorine Chem., 1981. Vol. 19. P. 35.
65. Husain S.Z., Plevey R.G., Tatlow J.C. // Bull. Soc. Chim. Fr., 1986. P. 891.
66. Knunyants I.L., Postovoi S.A., Delyagina N.I., Zeifman Yu.V. // Izv. Akad. Nauk SSSR. Ser. Khim., 1987. P. 2256-2261; Chem. Abstr., 1988. Vol. 109. 92244c.
67. Zeifman Yu.V., Postovoi S.A., Delyagina N.I. // Izv. Akad. Nauk SSSR. Ser. Khim., 1989. N 3. P. 738-740; Chem. Abstr., 1989. Vol. 111. 194224.
68. Postovoi S.A., Kargamanova E.M., Zeifman Yu.V. // Izv. Akad. Nauk SSSR. Ser. Khim., 1988. N 5. P. 1176-1180; Chem. Abstr., 1989. Vol. 110. 153710w.
69. Postovoi S.A., Zeifman Yu.V. // Izv. Akad. Nauk SSSR. Ser. Khim., 1988. N 4. P. 892-894; Chem. Abstr., 1989. Vol. 110. 23296e.
70. Kolenko I.P., Filyakova T.I., Zapevalov A.Ya., Mochalina E.P., German L.S., Polishchuk V.R. // Izv. Akad Nauk SSSR. Ser. Khim. 1979. N 3. P. 667-669; Chem. Abstr., 1979. Vol. 91. 38866d.
71. Aoyama H., Chiba Y. // Pat. 5945562 US (1999).
72. Ichihara K., Aoyama H. // Pat. 1085006 Europe (2001)
73. Skibida I.P., Sakharov A.M., Bakhmutov J.L., Denisenkow V.F., Martynova N.P. // Pat. 5495034 US (1996)
74. Sacharov A.M.,Bachmutov Yu.L.,Denisenkov V.F., Skoba I.P. // VI Conferene on Fluorine Chemistry. 26-28 June 1990. Novosibirsk. Abstracts. 1990. P. 106.
75. Kharchuk V.G., Shishmakov A.B., Volkov V.L., Petrov L.A. // Russ. J. Org. Chem., 1998. Vol. 34. N 2. P. 284-285; Chem. Abstr., 1998. Vol. 130. 3595.
76. Kharchuk V.G., Ilatovsky R.E., Saloutin V.I. // VI Conferene on Fluorine Chemistry. 26-28 June 1990. Novosibirsk. Abstracts. 1990. P. 109.
77. Zabolotskikh A.V., Pozdeaeva V.V., Zabolotskikh V.F. // 1th International Conference «Chemistry, Technology and Application of Fluorocompounds in Industry», May 30 - June 3, 1994. St. Petersburg. Russia. Abstracts. P4-26. P. 189.
78. Ichihara K., Miyamoto M., Baba N., Yoshii S., Homoto Y. // Jpn. Kokai Tokkyo Koho JP 2002053500 (2002); Chem. Abstr., 2002. Vol. 136. 167083t.
79. Nguyen T., Wakselman C. // Synth. Commun., 1990. Vol. 20. N 1. P. 97-99.
80. Kaneko I., Shinya P. // Asahi Garasu Kenkyu Hokoku, 1986. Vol. 36. N 2. P. 243-248; Chem.Abstr., 1987. Vol. 107. 134696t; Samezima S., Kaneko I. // Ger. Off. DE 3340141 (1984); Chem. Abstr., 1984. Vol. 101. 170715h.
81. Achilefu S., Mansuy L., Selve C., Thiebaut S. // J. Fluorine Chem., 1995. Vol. 70. P. 19-26; Theibaut S., Gerardin-Charbonnier C., Amos J., Selve C. // J. Fluorine Chem., 1995. Vol. 73. P. 179-184.
82. a. Zuczek C., Gerardin-Charbonnier C.,, Rocca S., Thiebaut S., Selve C. // J. Fluorine Chem., 1999. Vol. 99. P. 41-49.
b. Villaume B., Gerardin-Charbonnier C., Thiebaut S., Selve C. // Tetrahedron Lett., 2001. Vol. 42. P. 2305-2306.
c. Thenappan A., Puy M. // Pat. 5736012 U.S. (1998); Chem. Abstr. 1999. Vol. 128. 245458.
83. Nguyen T., Wakselman C. // J. Org. Chem. 1989. Vol. 54. N 23. P. 5640.
84. Szlavik Z., Tarkanyi G., Skribanek Z., Vass E., Rabai J. // Org. Lett. 2001. Vol. 3. N 15. P. 2365-2366; Chem. Abstr. 2001. Vol. 135. 210736b.
85. Hu C.M., Chen J. // J. Fluorine Chem., 1994. Vol. 67, P. 189.
86. Hu C.M., Qing F.L., Huang W.Y. // J. Org. Chem., 1991. Vol. 56, P. 2801.
87. Hu C.M., Tang X.Q. // J. Fluorine Chem., 1993. Vol. 61, P. 217.
88. Hu C.M., Qiu Y.L. // J. Chem. Soc., Perkin Trans. 1, 1992. P. 1569.
89. Takahashi M., Shuyama H. // Jpn, Kokai Tokkyo Koho JP 63 10742 (1988); Chem. Abstr., 1989. Vol. 110, 57128.
90. Dolbier W.R., Rong X.X., Bartberger M.D., Koroniak H., Smart D.E., Yang Z.-Y. // J. Chem. Soc., Perkin Trans, 2, 1998. N 2. P. 219-231.
91. Huang W.-Y., Zhuang J. // Chin. Chem. Lett., 1990. Vol. 1. N 3. P. 191-192; Chem. Abstr., 1991. Vol. 115, 135468x.
92. Wang Z., Lu X.Y. // Tetrahedron, 1995. Vol. 51. N 9. P. 2639-2658.
93. Feiring A.E. // J. Org. Chem., 1985. Vol. 50. N 18. P. 3269-3274.
94. Huang B.N., Haas A., Lieb M. // J. Fluorine Chem., 1987. Vol. 36, P. 49.
95. Nilsson N.H., Senning A. // Angew. Chem. Int. Ed. Eng., 1972. Vol.11, P. 295.
96. Tordeux M., Langlois B., Wakselman C. // J. Org. Chem., 1989. Vol. 54. N 10. P. 2452-2453.
97. Wakselman C., Tordeux M. // J. Chem. Soc., Chem. Commun., 1987. P. 1701.
98. Fuchigami T., Urata H., Obata Y. // Jpn Kokai Tokkyo Koho JP 63 152344 (1988); Chem. Abstr., 1989. Vol. 110, 7683y.
99. Fuchigami T., Urata H., Obata Y. // Jpn Kokai Tokkyo Koho JP 63 152343 (1988); Chem. Abstr., 1989. Vol. 110, 7682x.
100. Grottenmuller R., Knaup W., Probst A., Dullinger K // Appl. 10033255 Germany(2002)
101. Hu C.-M., Tu M.-H. // J. Fluorine Chem., 1991. Vol. 55, P.105.
102. Hu C.M., Qing F.L., Zhang H.G. // J. Fluorine Chem., 1990. Vol. 49, P. 275.
103. Hu C.-M., Yu Z.-H., Tu M.-H. // Youji Huaxue, 1994. Vol. 14. N 1. P. 44-48; Chem. Abstr., 1994. Vol. 121, 34758k.
104. Ledwith A., Russell P.J., Sutcliffe L.H. // J. Chem. Soc., Chem. Commun., 1971. N 16. P. 964-965.
105. Ozawa T., Setaka M., Kwan T. // Bull. Chem. Soc. Jpn., 1971. Vol. 44, P. 3473, Vol. 51. P. 357-379.
106. Hu L.Q., Huang W.Y. // Youji Huaxue, 1991. Vol. 11. N 2. P. 126-132; Chem. Abstr., 1991. Vol. 114, 246761m.
107. Dapremont-Avignon C., Calas P., Commeyras A., Amatore C. // J. Fluorine Chem., 1991. Vol. 51. P. 357-379.
108. Huang W.Y., Hu L.Q. // Acta Chim. Sin. (Engl. Ed.), 1989. N 1. P. 91-93; Chem. Abstr., 1990. Vol. 112, 118230j.
109. Dapremont-Avignon C., Calas P., Amatore C., Benefice-Malouet S., Cammeyras A. // J. Fluorine Chem., 1996. Vol. 77, P. 21.
110. Malkiats B.J. // J. Indian Chem. Soc., 1976. Vol. 53, P. 83.
111. Emelyanov G.A., Polyansky V.I., Berenblit V.V. // 1th International conference "Chemistry, technology and application of fluorocompounds in industry". May 30 - June 3, 1994. St. Petersburg, Russia. Abstracts. P 2-16. P Cherstkov V.F., Sterlin S.R., German L.S., Knunyants I.L. // Izv. Akad. Nauk SSSR. Ser. Khim., 1982. N 12. P. 2791.
112. Cherstkov V.F., Sterlin S.R., German L.S., Knunyants I.L. // Izv. Akad. Nauk SSSR. Ser. Khim., 1982. N 12. P. 2791..
113. Cherstkov V.F., Galachov M.V., Sterlin S.R., German L.S., Knunyants I.L. // Izv. Akad. Nauk SSSR. Ser. Khim., 1985. N 8. P. 1864-1868; Chem. Abstr., 1986. Vol. 105. 42306.
114. Cherstkov V.F., Sterlin S.R., German L.S., Knunyants I.L. // Izv. Akad. Nauk SSSR. Ser. Khim., 1982. N 8. P. 1917.
115. Millauer H. // Pat. 3128118 Ger. Offen (1983); Chem. Abstr., 1983. Vol. 98. 178738h.
116. Cherstkov V.F., Sterlin S.R., German L.S., Knunyants I.L. // Izv. Akad. Nauk SSSR. Ser. Khim., 1983. N 8. P. 1872.
117. Avetisyan E.A., Cherstkov V.F., Sterlin S.R., German L.S. // Izv. Akad. Nauk SSSR. Ser. Khim., 1990. N 3. P. 695.
118. Avetisyan E.A., Cherstkov V.F., Sterlin S.R., German L.S. // Izv. Akad. Nauk SSSR. Ser. Khim., 1989. N 9. P. 2073.
119. Cherstkov V.F., Sterlin S.R., German L.S., Knunyants I.L. // Izv. Akad. Nauk SSSR. Ser. Khim., 1985. N 1. P. 220.
120. Zapevalov A.Ya., Gorbunova T.I., Saloutin V.I. // Russ. RU 2035449 (1995); Chem. Abstr. 1996. Vol. 124. 145449s.
121. Chepik S.D., Belenkii G.G., German L.S. // Izv. Akad. Nauk SSSR. Ser. Khim., 1991. N 8. P. 1926-1928.
122. Hals L.J., Reid T.S., Smith G.H. // J. Am. Chem. Soc., 1951. Vol. 73. P. 4054.
123. Cherstkov V.F., Galachov M.V., Sterlin S.R., German L.S., Knunyants I.L. // Izv. Akad. Nauk SSSR. Ser. Khim., 1986. N 1. P. 119.
124. Cherstkov V.F., Galachov M.V., Mysov E.I., Sterlin S.R., German L.S. // Izv. Akad. Nauk SSSR. Ser. Khim., 1989. N 6. P. 1336.
125. Igumnov S.M., Shipigusev A.A., Lekontseva G.I., Gomzyakova O.D. // Russ. RU 2188187 (2002); Chem. Abstr. 2003. Vol. 139. 150065.
126. Deev L.E., Nazarenko T.I., Ponomarev V.G., Pashkevich K.I. // Usp. Khim. 1992. Vol. 61. P. 75-101.
127. Tonelli C., Gavezotti P., Strepparola E. // J. Fluorine Chem., 1999. Vol. 95. P. 51-70.
128. Marchionni G., Petricci S., Spataro G., Pezzin G. // J. Fluorine Chem., 2003. Vol. 124. P. 123-130.
129. Visca M., Silvani R., Marchionni G. // Chemtech, 1997. Vol. 27. N 2. P. 33-37.
130. Marchionni G., Spataro G., Strepparola E. // Pat. 5969192 US (1999).
131. Sheppard W.A., Sharts C.M. // Organic Fluorine Chemistry. Trans. In Jap. Yu. A. Cheburkov (Ed. I.L. Knunyants). M,.Mir. 1972. 480 P.
132. Udagawa A. // Jpn Kokai Tokkyo Koho JP 2001 240571; Chem. Abstr., 2001. Vol. 135. 212588d.
133. Kawa H. // Pat. 6479712 U.S. (2002).
134. Ishikawa N., Kobayashi Y. //Fluorine compounds. Chemistry and Application. Trans. In Jap. (Ed. Fokin A.V.). M, Mir. 1982. P. 210-220.
135. Zisman W.A. // Advance in Chemistry. No 43. I. American chemical society (1964).
136. Novoe v tekhnologii soedinenij ftora. // Pod red. N. Isikawa. M. : Mir, 1984, 592s.
137. Dehelean T., Valceanu R., Hu C.-M., Gusatu N., Xida J.-X. // Rev. roum. Chim. 2000. Vol. 45. N 4. P. 375-379
138. Prokhorov N.S., Petrov Yu.M., Aksyutin B.M., Belevtsev E.G., Bogomolov A.G., Zatylkin A.F., Sharin M.Yu. // The 2nd International conference "Chemistry, technology and applications of fluorocompounds". 23-26 Sept. 1997. St. Petersburg, Russia. Abstracts. P3-31. P. 148.
139. Kudimov V., Ermilova // The 3d International conference "Chemistry, technology and applications of fluorocompounds". 6-9 June 2001. St. Petersburg, Russia. Abstracts. P2-5. P. 70.
140. Ryabinin N.A., Irisova E.V. // The 2nd International conference "Chemistry, technology and applications of fluorocompounds". 23-26 Sept. 1997. St. Petersburg, Russia. Abstracts. P2-33. P. 105.
141. Yarosh A.A., Pryakhina T.A., Kotov V.M., Zavin B.G., Krukovsky S.P. // The 2nd International conference "Chemistry, technology and applications of fluorocompounds". 23-26 Sept. 1997. St. Petersburg, Russia. Abstracts. P2-24. P. 96.
Fluorine Notes, 2004, 37, 1-2
