Fluorine Notes, 2004, 36, 1-2
Perfluorinated Carboxylic Acids. Synthesis and Application.
Novosibirsk Organic Chemistry Institute of named after N.N. Vorojtsov Of RAS Siberian Department
Ac. Lavrentiev avenue, 9. Novosibirsk, Russia, 630090
E-mail : furin@nioch.nsc.ru
Dedicated to Professor Hermann-Josef Frohn on the Occasion of his 60th Birthday
In this article you will find data regarding perfluorinated carboxylic acids obtaining methods, in particular electrochemical fluorination of hydrocarbon derivatives in anhydrous hydrogen fluoride, perfluoroolefines and telomeric alcohols oxidizing, perfluoralkyliodides and carbon dioxide interaction in the presence of initiators. Some characteristics of perfluorinated carboxylic acids are discussed in this article. Using of these characteristics for creating of new semi-products, used for fluororganic synthesis is discussed here. Practical application examples of both perfluorinated carboxylic acids and semi-products based on them for creating of new generation fluorine materials are given in this article.
Table
of content
1. Introduction. The role of fluorine compounds in development and perfection
of materials for the new techniques.
2. The Synthesis of Perfluorinated carboxylic acids.
2.1. Electrochemical fluorination of carboxylic
acids and some of their derivatives in anhydrous hydrogen fluoride.
2.2. Oxidization processes
of linear and cyclic perfluorolefines and other fluorine-containing compounds.
2.2.1. The perfluorolefines
reactions with ozone.
2.2.2. Innovations in the oxidization processes of perfluorolefines' double
bond up to perfluoroalkancarboxylic acids.
2.2.3. The oxidization of telomeric alcohols and polyfluoroaromatic
compounds.
2.2.4. The transformations of perfluoroalkylhalogenides into perfluorocarboxylic acids under the initiators action.
2.2.5. The synthesis of perfluorocarboxylic acids out of other classes compounds.
3. The characteristics of perfluorocarboxylic acids and practical application of fluorine materials obtained on their basis.
3.1. Decarboxylation and decarbonization of perfluorocarboxylic acids.
3.2.
Surface-active materials on the base of perfluorocarboxylic acids derivatives.
3.3. Surface-active
agents as fire-extinguishing materials.
3.4. Compositions for treatment of articles surfaces,
rust-proofing coatings.
Conclusion
References
2.2.4. The transformations of perfluoroalkylhalogenides into perfluorocarboxylic acids under the initiators action.
Taking into account the availability of perfluoroalkylhalogenides, obtained in industry by telomerization
of tetrafluoroethylene in the presence of galogenides sources, the great attention was paid to
processes of their transformations to perfluorocarboxylic acids.
An interesting reaction was
found at perfluoroalkyliodides, perfluoroalkylbromides and perfluoroalkylchlorides heating in
DMF in the presence of bivalent tin (SnCl2/Al) [85] or lead (PbBr2/Al)
compounds [87,88], promoted by aluminium: the terminal fragment of CF2X was oxidized
and perfluoroalkylaldehydes monohydrates were formed at that [85-88].
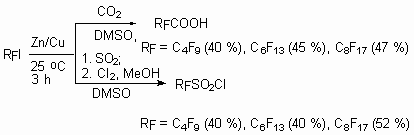
The reaction of perfluoroalkyliodides with CO2 in the presence of Zn in DMSO is of interest, as a result of which perfluorinated carboxylic acid is obtained. Thus the interaction of CF3(CF2)7I with CO2 in DMSO in the presence of zinc at 15 oC during 3 hours and after hydrolysis using aqueous NH3 results in formation of CF3(CF2)7CO2NH4 salt with the yield of 92 % [85-87]. The corresponding perfluoroalkylsulphochlorides are obtained in the analogous reaction with SO2 with further oxidization of primary product using chlorine [87].
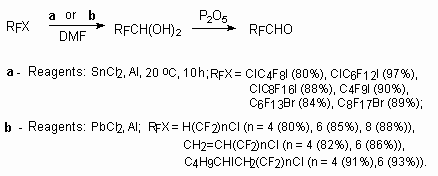
At oxidization of perfluoroalkyliodides R'I [ R' = linear or branched cyclic perfluoroalkyl] using air oxygen in the organic dissolvent (alcohol) in the presence of catalyst (metallic cuprum) the corresponding perfluorocarboxylic acids RCOOH are formed [88]. For example, ethyl perfluorooctanoate is obtained out of perfluorooctyliodide and copper powder in ethanol at heating conditions at 50 oC during 12 hours (yield is equal to 87%).
Fluorine containing aliphatic mono- and dicarboxylic acids were obtained by interaction of perfluoroalkyliodides or bromides and CO2 in the presence of complexes of VIII group transition metals with further hydrolysis of reaction products [89,90]. Thus, CF3(CF2)7CO2H is isolated with the yield equal to 40 % in reaction of CF3(CF2)7I with CO2 in DMF in the presence of Pd(PPh3)4 at 60 oC in autoclave with further hydrolysis. This points out the intermediate formation of perfluoroalkyl radical, which reacts with carbon dioxide.
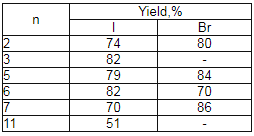
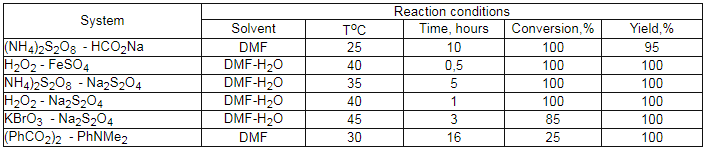
It turned out, that using of rongalite- NaHCO3 system in bipolar aprotic dissolvents (MeCN, DMF) in reaction with perfluoroalkyliodides and perfluoroalkylbromides, containing 3-12 carbon atoms (Cl(CF2)nI ,where n = 4, 6, 8; CF3(CF2)nI, where n = 5-7; Cl(CF2)n NBr, where n = 4, 6) allows to obtain perfluorocarboxylic acid sodium salts with high yields (Table 3) [91-97]. This synthesis method of perfluorocarboxylic acids is much convenient compare to the known method of their obtaining, based on electrochemical fluorination and their derivatives.
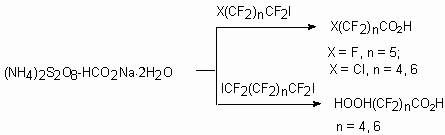
 |
If the process is carried out in the presence of alkenes and source of CO2 then forming
due to perfluoroalkyl radical addition according to multiply bond intermediate radical will react
with CO2 with forming of carboxylic acid. When using alcohol as dissolvent the product
is corresponding ester of acid. Thus, C8F17CH2CH(Bu)CO2Et
was obtained with the yield equal to 67% by interaction of hex-1-ne and C8F17I.
in the presence of K2CO3 and dichloro-bis-(triphenylphosphine)palladium
in ethyl alcohol at 80 oC during 12 hours [98,99].
Such reductive-oxidative systems as (NH4)2S2O8-HCOOH-H2O
allow to transform selectively perfluoroalkyliodides,  ,
,
 -diiodoperfluoroalkanes compounds like RFCCl3, RFCFBrCl, RFCBr2Cl,
RFCFCl2 and others into corresponding polyfluorocarboxylic acids and
diacids (table 3) [101-103].
-diiodoperfluoroalkanes compounds like RFCCl3, RFCFBrCl, RFCBr2Cl,
RFCFCl2 and others into corresponding polyfluorocarboxylic acids and
diacids (table 3) [101-103].
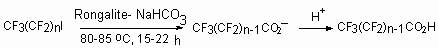
Table 3.
The transformation of polyfluoroalkylhalogenides into corresponding acids under the influence of (NH4)2S2O8-HCOONa*2H2O system in DMF [95].
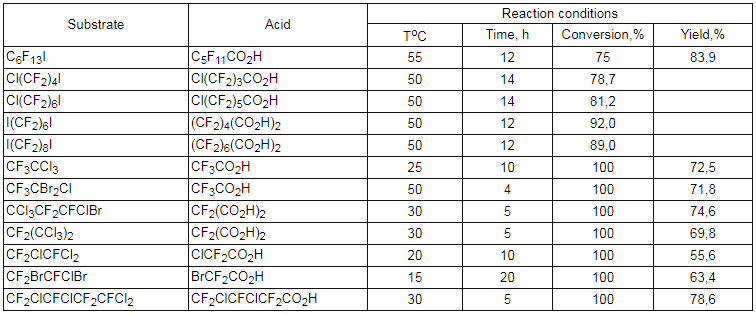 |
Sodium salts of perfluoroalkansulphinic acid together with oxidation-reduction reagent (CH4)2S2O4 were used to obtain corresponding carboxylic acids [104-106].
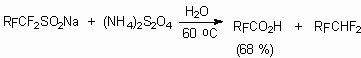
Analoqously AgNO3-HCO2Na system catalyzes reaction in aqueous acetonitril [107].
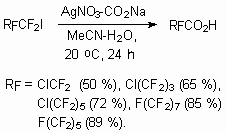
1,1,1-trifluorotrichloroethan (CCl3CF3) can produce trifluoroacetic acid in the presence of different oxidizing agents (table 4) [102]. The reaction must go according to the following scheme [102].
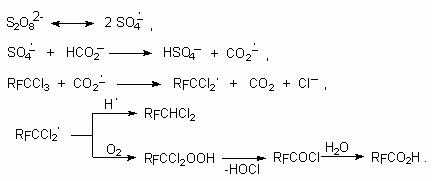
Table 4. Conversion of CF3CCl3 into trifluoroacetic acid in the presence of different oxidation-reduction systems [102].
In this case the generation of polyfluoroalkyl radical under the influence of CO2. - anion-radical the key stage. It is known, that other anion-radicals can carry out such transformations.
Thus it is stated [108,109], that corresponding acids (yields up to 50%) are formed out of perfluorobutyliodide,
perfluorooctyliodide and  ,
,
 -diiodoperfluorobutane under the influence of electrochemically generated molecular oxygen O2-. anion-radical.
-diiodoperfluorobutane under the influence of electrochemically generated molecular oxygen O2-. anion-radical.

The reaction goes according to the following scheme:
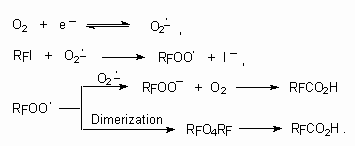
In the presence of (NH4)2S2O8 the corresponding perfluoroalkancarboxylic acids are the products of reaction of perfluoroalkansulphinic acid sodium salt with alkenes along with compounds, possessing multiply bonds, that can be explained by hydrogenolysis C-Hal [104-106,108-110].
It should be noted, that for the first time this approach was realized for reactions of some aromatic
compounds with perfluoroalkyliodides and equimole amount of peroxide. We can suppose, that optimal
conditions for carring out of such reactions will be found and they will take their place in
the processes of perfluoroalkylation using perfluoroalkyliodides.
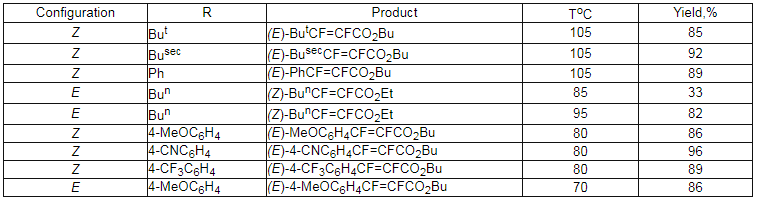
The interaction of (E)-
and (Z)-1,2-difluoro-1-iodalkens and (E) and (Z)- ,
,
 -difluoro-
-difluoro- -iodstiroles
with carbon oxide in the presence of catalyst Pd(PPh3)2Cl2 at 70-105 oC and in the presence of alcohol and trialkylamine form esters of carboxylic
acids. (table. 5). It should be noted, that in these reactions the complete changing of configuration
takes place.
-iodstiroles
with carbon oxide in the presence of catalyst Pd(PPh3)2Cl2 at 70-105 oC and in the presence of alcohol and trialkylamine form esters of carboxylic
acids. (table. 5). It should be noted, that in these reactions the complete changing of configuration
takes place.
Table 5. The interaction of perfluoroalkenyliodides and carbone oxide in the presence of catalyst.
It is shown [107], that electrolysis of perfluorobutyliodide at carbon cathode in the presence of oxygen in dimethylformamide results in formation of perfluorobutanoic acid. In this process the key part plays forming at current action oxygen anion-radical, which influencing on perfluorobutyliodide produces C4F9O2 radical.
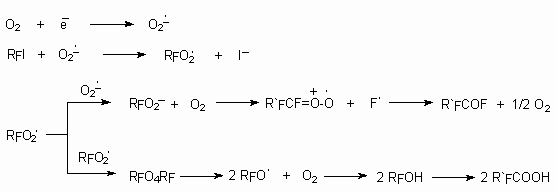
Perfluoroalkyliodides are oxidized by oxygen in the presence of catalysts (copper) in the medium
of organic solvent up to perfluorocarboxylic acids [110]. For example, perfluorooctyliodide in
ethyl alcohol in the presence of metal copper at 50 oC in 12 hours results in formation
of ethyl perfluorooctanoate with the yield of 87%. [110].
2.2.5. Perfluorocarboxylic acids synthesis out of other compounds classes.
Terminal fluoroolefines of aliphatic series in non-catalytic reaction with SO3 form
only  -sultones,
while at catalysis using boron compounds (BF3) the formation of unsaturated fluorosulphate
in big amounts is observed [112]. At the same time the catalysis of SbF5 reactions
of internal perfluoroolefines with SO3 produces exclusively unsaturated fluorosulphates
[113,114].
-sultones,
while at catalysis using boron compounds (BF3) the formation of unsaturated fluorosulphate
in big amounts is observed [112]. At the same time the catalysis of SbF5 reactions
of internal perfluoroolefines with SO3 produces exclusively unsaturated fluorosulphates
[113,114].
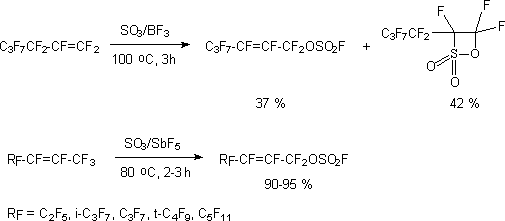
The reaction is not accompanied by migration of double bond; the stereoisomeric composition corresponds exactly to the stereoisomeric composition of reacting olefine, at trans- isomers react faster, than cis-isomers.
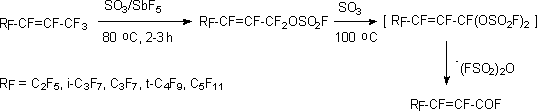
Polyfluoroalkylfluorosulphates are colorless, mobile, resistant to hydrolysis liquids (n<8)
or solid compounds (n>8), stable at long-term storage. Their most important property is
their changing for  -hydroperfluorocarboxylic
acids fluoroanhydrides at processing using metal fluorides. This is used as polyfluorinated
carboxylic acids obtaining method [52].
-hydroperfluorocarboxylic
acids fluoroanhydrides at processing using metal fluorides. This is used as polyfluorinated
carboxylic acids obtaining method [52].
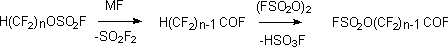
The further replacement of hydrogen for FSO2O group results in formation of  -fluorosulphonyl-oxy-perfluoroacylfluorides,
which there are the initial products for ion-exchange membranes. In case of SO3 [SbF5] excess the saponification of CF3 groups and formation of corresponding
carboxylic acids take place [114].
-fluorosulphonyl-oxy-perfluoroacylfluorides,
which there are the initial products for ion-exchange membranes. In case of SO3 [SbF5] excess the saponification of CF3 groups and formation of corresponding
carboxylic acids take place [114].
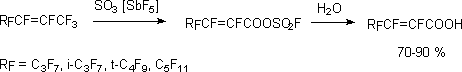
It turned out, that perfluoroalkenoyl fluorides are also formed at interaction of perfluoroalken-2-ylfluorosulphates
with CsF according to sulfur atoms, and  ,
,
 -unsaturated perfluorocarboxylic acids esters are formed at alcohol influence in the presence
of KF [111,116].
-unsaturated perfluorocarboxylic acids esters are formed at alcohol influence in the presence
of KF [111,116].
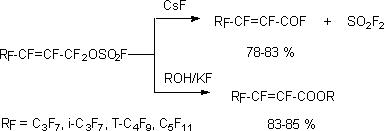
It should be kept in mind, that the reaction like this with linear alkenylfluorosulphates, containing
in  -position
voluminous perfluoroalkyl groups, results not in formation of corresponding acids, but in
formation of two perfluoroolefines mixture [117,118].
-position
voluminous perfluoroalkyl groups, results not in formation of corresponding acids, but in
formation of two perfluoroolefines mixture [117,118].
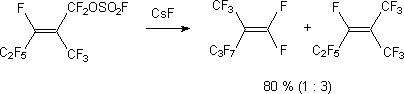
At alkali metals halogenides action on perfluoroalken-2-ylfluorosulphates the products of direct FSO2O-group replacement for halogenide ions [118,119].
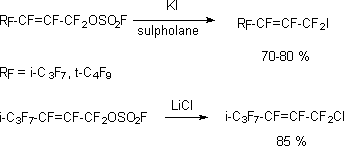
For example, trifluoroacrylic acid haloidanhydride is obtained out of perfluoroallylfluoro-sulphate (CF2=CFCF2OSO2F) by action of KBr or NaBr in ether (diglyme, sulpholane) [120].
The important moment of carboxylic acid fluoroanhydride reactivity is their changing into salts under the action alkali metals fluorides, such salts contain perfluoroalkoxi group, able to substitute easily moving groups in organic molecules. For example, perfluoroalkylallyl ethers became available after working out of chemistry and technology of perfluoroallylfluorosulphates due to condensation of the last mentioned with perfluoroalkoxy-anion according to the scheme [111] :
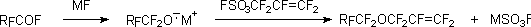
Perfluoroallyl cations, generated by action of SbF5 on internal perfluorolefines transform into corresponding perfluorinated acids fluoroanhydrides. Thus, 1-trifluoromethoxyperfluoroallyl cation turns transform into trifluoroacryloate fluoride, an important semi-product of fluororganic synthesis [121].
3. Characteristics of perfluorocarboxylic acids and practical application of fluoromaterials obtained on their base.
3.1. Decarboxylation and decarbonylation of perfluorocarboxylic acids.
Among characteristics of perfluorocarboxylic acids we should first of all mention the transformation of carboxylic group. Depending on conditions during these processes different products are obtained. At that perfluoroalkyl fragment can also participate in these processes. Thus, polyfluorinated carboxylic acids H(CF2CF2)n COOH (n = 1-5) at alkali action transform into terminal perfluoroolefines with different length of perfluoroalkyl substituent. The obtaining method of terminal perfluoroolefines is based on this.
.gif)
Perfluorocarboxylic acids natrium salts can be used, at that the yield is stable and almost quantitive [122].
.gif)
Best results are obtained at carrying out decarbonylation of perfluorocarboxylic acids halogenanhydrides and their alkyl ethers in the presence of catalysts (oxides of metals Mg, Cr, Ba, Zn, Al, Ni, Si), promoted by using of 20-50 % alkali metals halogenides at 100-300 oC. The yield of terminal perfluoroolefines reaches 95 % [108].
The reactions of 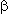 -unsaturated perfluorocarboxylic acids, going with their decarboxylation, open the way to synthesis of different compounds of ethylene and acetylene series [123]. For example, thermal decomposition of Na perfluoro-
-unsaturated perfluorocarboxylic acids, going with their decarboxylation, open the way to synthesis of different compounds of ethylene and acetylene series [123]. For example, thermal decomposition of Na perfluoro-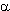 ,
,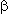 -unsaturated perfluorocarboxylic acids carboxylates in ethylene glycol results in formation of 1-hydroperfluoroalkenes-1, while the one (decompositon) of carboxylates Ag of these acids results in formation of perfluorinated
-unsaturated perfluorocarboxylic acids carboxylates in ethylene glycol results in formation of 1-hydroperfluoroalkenes-1, while the one (decompositon) of carboxylates Ag of these acids results in formation of perfluorinated 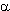 -diens [123,124].
-diens [123,124].
.gif)
Thermal decomposition of copper perfluoroalkenylcarboxylates Cu2+ in high-boiling organic solvents, for example in N- methylpirrolidone or sulfolane, results in formation of mixture of 1,3-diens and 1-hydroperfluoroalkens-1 [124].
.gif)
Halogenides and esters of perfluorocarboxylic acids are subject to decarboxylation at 100-300 oC in the presence of catalysts (oxides of magnesium, calcium, barium, zinc, nickel) resulting in formation of perfluoroolefines or perfluoroalkylvinyl ethers with the yield close to 95% [125].
Terminal perfluorolefines can be semi-products to obtain:
1. polyfluoroalkansulpho-acids and on their base the surface active materials and electrolytes for lithium batteries and rechargeable accumulators,
2. new chelation for salts of rare elements,
3. creation of high-temperature liquid dielectrics, heat-transfers and hydraulic liquids.
.gif)
At the same time at perfluorocarboxylic acids fluoroanhydrides passage over Al2O3 decarbonylation with perfluoroalkanes formation is going. It should be noted, that at these conditions chloro- and bromoanhydrides of corresponding acids do not change using of activated carbon is required to carry out this process, at that chloro-and bromperfluoroparafines are formed [126].
.gif)
If in the system there are sources of halogenide-ions (for example, alkali metals halogenides, haloids etc.) then the thermolysis of perfluocarboxylic acids chloroanhydrides, conducted in flow reactor at atmospheric pressure and 250-350 0C in the presence of potassium iodide, will result in formation of iodoperfluoroalkanes with the yield of 80-85%, and heating of mixture of perfluorinated acids fluoroanhydrides and bromine at 300-450 oC at activated carbon will result in formation of bromoperfluoroalkanes [126].
Thermal reactions of sodium and potassium salts of mono- and di-perfluorocarboxylic acids (temperatures 150-250 0C) result in processes of decarboxylation and formation of hydrocarbons with end group CF2H. By the example of thermal decarboxylation of perfluoropolyetherdicarboxylic acids with the structure ROOCCF2-RF- CF2COO, which was produced at oxidizing polymerization tetrafluoroethylene with O2 at UV-irradiation, were studied the kinetics and reaction products.

Perfluorinated carboxylic acids are presented as reagents for introduction of RFCO or RFCOO fragments and they are rather deeply and thoroughly studied [131]. We will point out, for example, acylation of compounds with active hydrogen atom, that are used for temporarily blocking of OH and NH2 groups in carbohydrateū and peptides, acetylation of aromatic compounds according to Friedel-Crafts with formation of perfluoroalkylarylketones, reactions with metalo-organic compounds with formation of perfluoroalkyl-containing tertiary alcohols, addition to olefins and acetylene reactions, including different unsaturated fluorinated systems in the presence of fluoride- ions for synthesis of perfluorinated dialkylketones. This important synthetic achievement, using fluorine carb-anion intermediate products, for example:
.gif)
In connection with high need of partly fluorinated alcohols their obtaining approach based on their esters of perfluorocarboxylic acids is developing. Thus, reduction of esters of carboxylic acids like RFCOOR [ RF = CF3(CF2)m(CH2)n (m = 0-20, n = 0-5), H(CF2)m(CH2)n (m = 1-20, n = 0-5), (CF3)2CF, CF2=CF, CF2=CFCF3; R = Me, Et, n-Pr,
i-Pr] by NaBF4 action in teterahydrofurane produce the corresponding alcohols [132].
For example, CF3(CF2)6CH2OH alcohol is obtained out of methyl perfluorooctanoate with the yield equal to 98.3 % (100 % conversion). Like this the reduction of perfluoro(hexyldecylacetate) is carried out by action of NaBF4 in the special solvent up to perfluoro-1H,1H-2-hexyldecanol [133].
Thereby, there are no special difficulties at target setting of carbonyl-containing compound synthesis based on perfluorocarboxylic acids. We consider discussion of these questions inexpedient, and we'll dwell on practical application of these fluorine materials.
To be continued
Fluorine Notes, 2004, 36, 1-2
