Fluorine Notes, 2004, 35, 1-2
Perfluorinated Carboxylic Acids. Synthesis and Application.
G.G.Furin
Novosibirsk Organic Chemistry Institute of named after N.N. Vorojtsov Of RAS Siberian Department
Ac. Lavrentiev avenue, 9. Novosibirsk, Russia, 630090
E-mail : furin@nioch.nsc.ru
Dedicated to Professor Hermann-Josef Frohn on the Occasion of his 60th Birthday
In this article you will find data regarding perfluorinated carboxylic acids obtaining methods, in particular electrochemical fluorination of hydrocarbon derivatives in anhydrous hydrogen fluoride, perfluoroolefines and telomeric alcohols oxidizing, perfluoralkyliodides and carbon dioxide interaction in the presence of initiators. Some characteristics of perfluorinated carboxylic acids are discussed in this article. Using of these characteristics for creating of new semi-products, used for fluororganic synthesis is discussed here. Practical application examples of both perfluorinated carboxylic acids and semi-products based on them for creating of new generation fluorine materials are given in this article.
Table of content
1. Introduction. The role of fluorine compounds in development and perfection of materials for the new techniques.
2. The Synthesis of Perfluorinated carboxylic acids.
2.1. Electrochemical fluorination of carboxylic acids and some of their derivatives in anhydrous hydrogen fluoride.
2.2. Oxidization processes of linear and cyclic perfluorolefines and other fluorine-containing compounds.
2.2.1. The perfluorolefines reactions with ozone.
2.2.2. Innovations in the oxidization processes of perfluorolefines' double bond up to perfluoroalkancarboxylic acids.
2.2.3. The oxidization of telomeric alcohols and polyfluoroaromatic compounds.
2.2.4. The reactions of perfluoroalkylhalogenides under influence of initiators.
2.2.5. The synthesis of perfluorocarboxylic acids out of other classes compounds.
3. The characteristics of perfluorocarboxylic acids and practical application of fluorine materials obtained on their basis.
3.1. Decarboxylation and decarbonization of perfluorocarboxylic acids.
3.2. Surface-active materials on the base of perfluorocarboxylic acids derivatives.
3.3. Surface-active agents as fire-extinguishing materials.
3.4. Compositions for treatment of articles surfaces, rust-proofing coatings.
Conclusion
References.
2.1. Electrochemical fluorination of carboxylic acids and some of their derivatives in anhydrous hydrogen fluoride.
If carboxylic acid posess N,N- dialkylamino group as substituent, then the direction of fluorination process doesn't change and both fluoroanhydrides of corresponding perfluorinated acids and heterocyclic compounds are formed. (table. 2) [27-31].
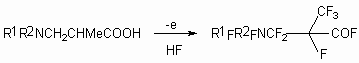
These compounds are used as semi-products for creating of surfactants and monomers for fluorine containing polymers, medications.
The yield of target products depends on carbon chain type. [32].
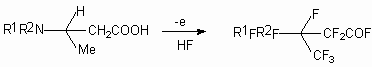
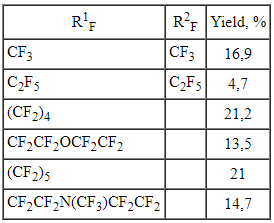
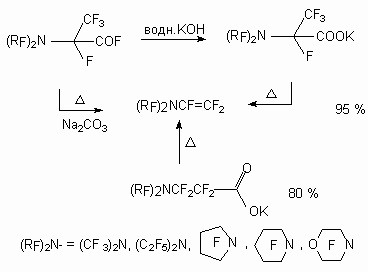
It's interesting to note, that such perfluorinated compounds were used for synthesis of perfluoro-(N-vinyl amines) [33-35]. During polymerization the last mentioned ones produce films, which let pass selectively gases of certain type, this is used for gas separation, in particular for air oxygen enriching.
At electrochemical fluorination of 2- pyrrolidine propionic acid with further heating of fluorination product at 100-150 oC in the presence of basis the perfluorinated N-vinyl substituted cyclic amines are formed [33-35]. These compounds can be used to obtain surfactants, dyes, medications. The detailed studying of this process showed [36-38] the influence of both process carrying out conditions and carbon chain structure on the formation of N-vinyl amines.
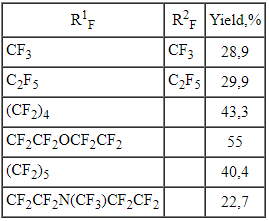
Table 2. The results of N,N-dialkylamino substituted carboxylic acids electrochemical fluorination [27].
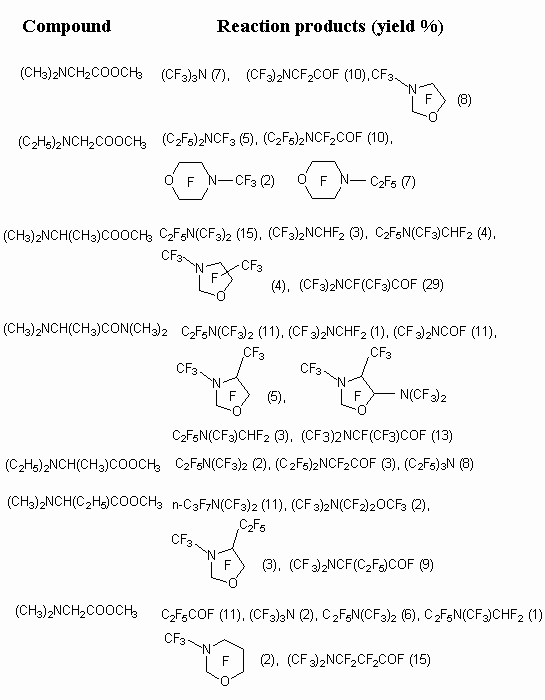
The yield of perfluorocarboxylic acids fluoroanhydrides depends on carbon chain length, connected with nitrogen atom. The following order regarding increasing of target product is determined [39].
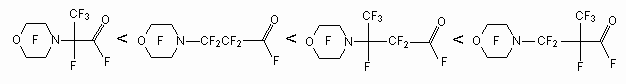
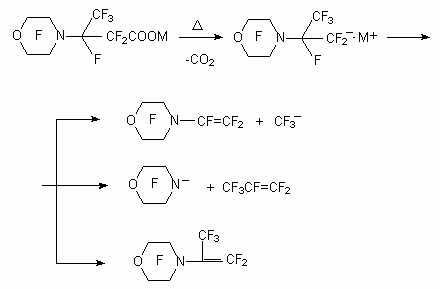
The behavior of carboxylic acids esters, containing cyclic amine as substituent in  -position, is analoguos at electrochemical fluorination. The formation of entirely fluorinated compounds takes place, it goes with complete retaining of carbon skeleton and products of cyclic system reducing.[32, 40,41].
-position, is analoguos at electrochemical fluorination. The formation of entirely fluorinated compounds takes place, it goes with complete retaining of carbon skeleton and products of cyclic system reducing.[32, 40,41].
At the same time if this group is in  -position, then
-position, then  - disintegration of carbon chain occurs (more rarely the opening of C-N bond) : the formation of perfluorinated trialkylamines occurs (current density 3.3 A/ dm2, voltage 5.9-6.1 V, temperature 7-9 oC) [42-44].
- disintegration of carbon chain occurs (more rarely the opening of C-N bond) : the formation of perfluorinated trialkylamines occurs (current density 3.3 A/ dm2, voltage 5.9-6.1 V, temperature 7-9 oC) [42-44].
The result of electrochemical fluorination of cis-2,6-dimethyl morpholine substituted carboxylic acids is the formation of fluoroanhydrides of corresponding acids along with other fluorination products [45-47].
The electrochemical fluorination of corresponding piperazine-N,N'-di- -propionic acid results in formation of the fluoroanhydride of perfluorinated piperazine-N,N'-di-
-propionic acid results in formation of the fluoroanhydride of perfluorinated piperazine-N,N'-di- -propionic acid [48,49]. These compounds are used as lubricating oils, water- and oilrepellent agents and medicines.
-propionic acid [48,49]. These compounds are used as lubricating oils, water- and oilrepellent agents and medicines.
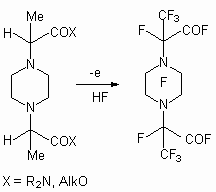
The electrochemical fluorination of alkoxycarboxylic acids results in formation of perfluorocarboxylic acid perfluoroalkyl esters (Electrochemical fluorination conditions: voltage 6.5 V, temperature 10 oC, current density 0.01-0.02 A/dm2, Ni- anode, steel-3 as cathode) [50].
Higher perfluorocarboxylic acids [51] are obtained by telomeric alcohols H(CF2CF2)nCH2OH , n = 2-5) electrochemical fluorination in anhydrous hydrogen fluoride. The obtained acids had found their application at emulsion polymerization of tetrafluoroethylene.
It should be noted, that direct fluorination of carboxylic acid derivatives by element fluorine have to be carried out in the solution of perfluorinated solvent fluorine-resistant. In the recent years the satisfactory results regarding both the fluorination process and target product yield are obtained. Thus the fluorination of succinic acid dimethyl ester using element fluorine deluted with nitrogen (1:4) in perfluoro-(N-methylmorpholine) at 20 oC results in formation of succinic acid perfluorinated dimethyl eater with the yield of 75%.
2.2. The oxidization processes of linear and cyclic perfluorolefines and other fluorine containing compounds.
2.2.1. The Reactions of Perfluoroolefines with ozone.
One of the perfluorolefines oxidization methods is their reaction with ozones. Thus the oxidization of hexafluoropropylene by ozone at 23 oC and pressure equal to 700 mm Hg. results in formation of COF2and CF3COF with quantity yield [52]. This process can be considered as an obtaining method of trifluoroacetic acid out of hexafluoropropylene. The process is convenient to held in acidic medium. [53].
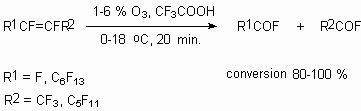
In the presence of CF3CH2OH the oxidization of internal polyfluorolefines passes by ozone with formation of corresponding ester [54].
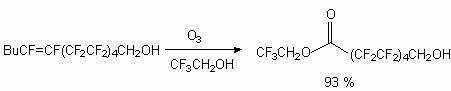
Thus, in the works [55,56] it is shown, that ozonolysis of cyclic and acyclic perfluoroalkene in freon 113 with further restoring of ozonolysis peroxide products in the atmosphere of hydrogene over palladium catalyst with the yield of 70-90% results in formation of corresponding perfluorocarboxylic acids that is perfluoroheptanoic, perfluorohexanoic and 2-trifluoromethylperfluoropropionic acids. The ozonolysis of 1-methoxyperfluorocyclobutene in the freon 113 with further reduction results in formation of perfluorosuccinic acid methyl ester [55].
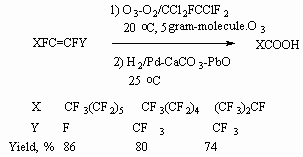
The ozone action on perfluoroct-1-ene and perfluoroct-2-ene (mixture of Z-and E- isomers in proportion 1.0:2.4).in the trifluoroacetic acid medium by oxygen-ozone (1-6%O3) at 0 - -18 oC results in formation of mixture (1:1) 3,3,5-trifluoro-5(perfluorohexyl)- and 3,5-trifluoro-3(trifluoromethyl)-5(perfluoropentyl)-1,2,4-trioxolanes correspondingly [57].
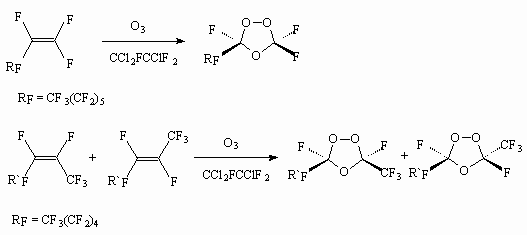
Such ozonides are effective catalysts of fluoromonomers and acrylates polymerization and co-polymerization at -10 - 50 0oC and pressure of 3 *10 kPa [58].
Hydrogenation of ozonides over palladium catalyst results in perfluoroalkancarboxylic acids.
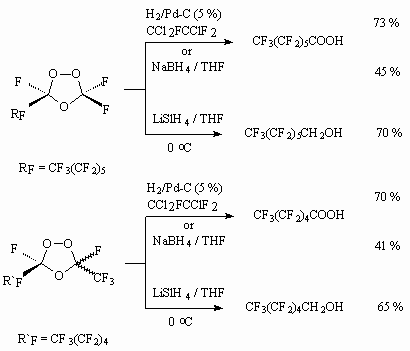
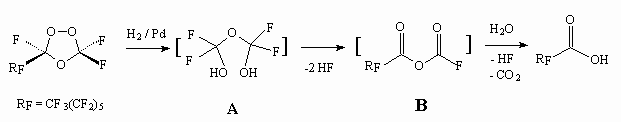
If the action of sodium borane stops at the stage of perfluoroalkancarboxylic acids formation, then the application of lithium aluminum hydride will result in formation of corresponding perfluoroalkylcarbinols. It should be noted, that carrying out of perfluorolefines ozonolysis in the alcohols' medium results in formation of corresponding perfluoroalkancarboxylic acids [57]. The authors of this work suppose, that reduction decomposition of peroxide bridge results in intermediate A, which looses anhydrous hydrogen fluoride because of unstable  -fluorine hydrin groups with formation of anhydride B, easily hydrolyzing up to acid.
-fluorine hydrin groups with formation of anhydride B, easily hydrolyzing up to acid.
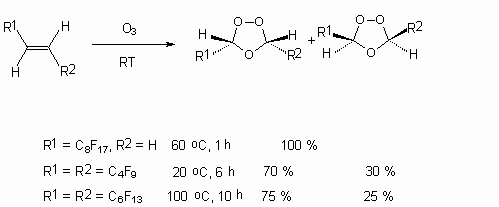
In case of partly fluorinated olefins interaction with ozone not corresponding carboxylic acids are formed, but the reaction stops at the stage ozonides obtaining [59].
Low temperature (77-280 K) ozonolysis of teterafluoroethylene, hexafluoropropylene and its dimer and trimer is carried out by direct ozone action without dissolvent, at that the ozonides are formed, which are effective as initiators of teterafluoroethylene and other fluoromonomers polymerization [60].
Ozonolysis of the 1-metoxyperfluorocyclobut-1-ene in freon 113 and further hydrogenation (H2 / Pd-CaCO3, 20 oC, H2O) results in formation of 3- metoxycarbonyl-2,2,3,3-tetrafluoropropionic acid [61]. Besides, the further thermolysis of this acid produces its dimer.
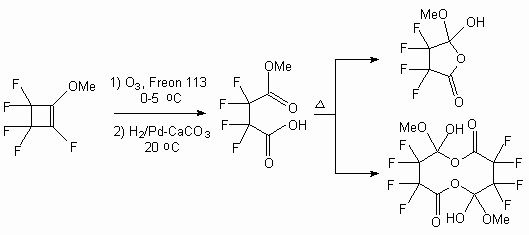
Fluorinated cyclohexanes are also oxidized by ozone with cycle opening and formation of corresponding acids [62].
2.2.2. New in the Oxidization Processes of Perfluoroolefines Double Bond up to Perfluoroalkancarboxylic Acids.
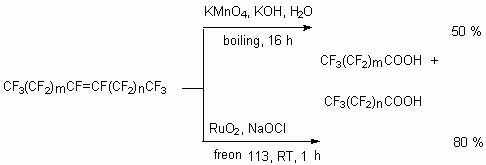
The oxidization processes of perfluoroalkyl ethylene and propylene derivatives proceed mostly easy, that is studied rather widely. The oxidization of internal perfluoroolefines proceed at more severe conditions. Thus, acting of such oxidizers as potassium permanganates and ruthenium tetraoxide affect the multiply bond and, as a rule, after hydrolysis two perfluorinated carboxylic acids are formed [63].
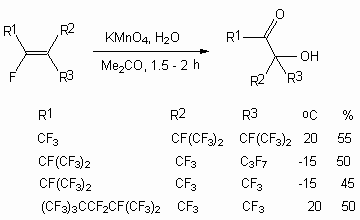
It is known, that when only the perfluoroalkyl substituents have a multiply bond, the oxidization using potassium permanganate will result exclusively in formation of diol [64].
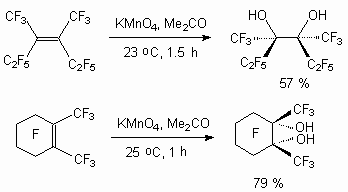
Analogously the heating of R2C=C(CF3)2 [R = CF3, C2F5] together with KMnO4 in aqueous acetone produces diol HOCR2C(CF3)2OH, and perfluoro(1-ethyl-2-isopropylcyclopentene) results in formation of diketone C2F5C(O)(CF2)4C(O)CF(CF3)2 [64]. At KMnO4 action dialkyl substituted fluoroolefines of C4 - C7 type and perfluorocyclohexene turn into  -diketones with 40-50% yield [64,65]. Trans-isomers much easily react with KMnO4, than the corresponding cis-isomers.
-diketones with 40-50% yield [64,65]. Trans-isomers much easily react with KMnO4, than the corresponding cis-isomers.
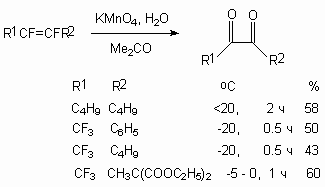
At the same time the oxidization of trialkyl substituted fluorolefines using the same reagent results in formation of  -hydroxyketones [66-69].
-hydroxyketones [66-69].
Chromium trioxide in fluorosulphonic acid shows strong oxidizing properties. Thus this oxidizing system influencing hexafluoropropylene produces hexafluoropropylene oxide with the yield of 55% [70]. At the same time the addition of Cr2O3 to this system results in formation of fluorosulphinate pentafluoroacetonyl.
The oxidization of perfluoroalkylethylens (RCH=CH2, R = perfluoroalkyl C2-C14) multiply bond goes most easily at oxidization using sodium hypochlorite NaOCl in the presence of RuO2 *7H2O in organic dissolvent (tert-buthanol, MeCN, diglyme, 1,3-dioxane). For example, at 30 oC and atmospheric pressure C8F17CH=CH2 turns into perfluoroctylcarboxylic acid C8F17COOH in 3 hours with the yield of 98 % [71].
2.2.3. The Oxidization of Telomeric Alcohols.
Telomeric alcohols like H(CF2CF2)nCH2OH (where n = 1-8) can be oxidized up to corresponding  -hydroperfluorocarboxylic acids, playing a great part in the creation of different fluorine materials of practical purpose. The oxidization of telomeric alcohols is carried out by action of potassium permanganate, nitrogen oxides, Chromium anhydride, chlorine at 100-140 oC and UV irradiation. However these methods have essential disadvantages and production technologies on their base are not developed. There by researchers still keep an eye on telomeric alcohols oxidization processes.
-hydroperfluorocarboxylic acids, playing a great part in the creation of different fluorine materials of practical purpose. The oxidization of telomeric alcohols is carried out by action of potassium permanganate, nitrogen oxides, Chromium anhydride, chlorine at 100-140 oC and UV irradiation. However these methods have essential disadvantages and production technologies on their base are not developed. There by researchers still keep an eye on telomeric alcohols oxidization processes.
Thus, telomeric alcohols (C1-16) are oxidized by nitric acid in the presence catalysts, which are the oxides and salts of ferrum, nickel, cuprum and vanadium [72]. At oxidization of H(CF2)8CH2OH using 55 % nitric acid together with synchronous oxygen introduction at 125 oC and pressure of 0.8 MPa in the presence of FeCl2*.nH2O in 7 hours at 100 % alcohol conversion H(CF2)8COOH is formed with yield of 99.6 % [72].
Catalytic system, containing complexes of divalent cuprum, catalyses selective oxidization of telomeric alcohols up to perfluorocarboxylic acids [73,74]. This method is especially effective for alcohols with long carbon chain (C8-14). The process goes in alkaline medium, which is necessary for conversion of alcohol into anionic form, that greatly facilitates activization of molecular oxygen at low temperatures.
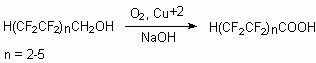
Obviously, the participation of cuprum complexes in electrone transferring from alcoholates ions onto oxygen allows the reaction to go according to thermodinamically advantageous dielectron mechanism, that determines abnormally high rates of oxidization. The reaction rate almost doesn't depend on molecular mass of oxidized alcohols in the interval n = 1 - 6. The variation of reaction carrying out conditions (temperature, dissolvent, ligand, concentration of alkaline and catalyst etc.) allows to oxidize selectively (with 100%) the telomeric alcohols. For example, HCF2CF2CH2OH is oxidized using molecular oxygen in the presence of cuprum catalyst and alkaline agent (in the presence of 5* 103 M CuCl2 solution and 1.10-2 ? ortho-phenanthroline solution in isobutyl alcohol and NaOH at 45 oC, 6.5 h) with the yield of 73.8 % up to HCF2CF2COOH (conversion 50.5 %).
The oxidization of telomeric alcohols H(CF2)nCH2OH (n = 2, 4, 6) in liquid phase using air oxygen at 350 oC in the presence of V2O5 results in formation of H(CF2)nCH(OH)2 with the yield equal 74-93 % [75]. Other catalysts may be also used [76].
The authors of works [77-79] had developed the approach to the synthesis of 3-chlorotetrafluoropropionic acid using photochemical chlorination of borate and phosphate esters of telomeric alcohol HCF2CF2CH2OH.
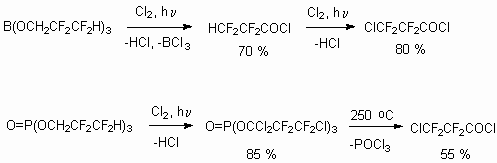
The chlorine action is carried out at UV-irradiation (lamp 100-400 watt) at 25-110 oC, at that further substitution of  - and
- and 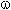 - atoms of alcohol hydrogen can happen. The decomposition of chlorinated borate passes at reaction conditions up to acid chloroanhydride, while chlorinated phosphates require higher temperatures (180-250 oC). At the same time the chlorine action on telomeric alcohols in the presence of catalyst results in formation of 1-chloroperfluoroalkanes [78].
- atoms of alcohol hydrogen can happen. The decomposition of chlorinated borate passes at reaction conditions up to acid chloroanhydride, while chlorinated phosphates require higher temperatures (180-250 oC). At the same time the chlorine action on telomeric alcohols in the presence of catalyst results in formation of 1-chloroperfluoroalkanes [78].
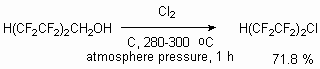
The telomeric alcohols have two reaction centers, according to which the reactions can be carried out. The telomeric alcohols reactivity in reactions, passing affecting the alcoholic group is discussed above. At the same time the existence of CHF2 fragment at the end of carbon chain allows to expect the turning with its active participation. First of all, the existence of fluorine atoms and the influence of CF2fragment next to it results in acidity increasing and thereafter the increasing of carb-anion generation possibility. Indeed, the action of two gram-molecules of alkyllithium results in multiply terminal bond formation. It allows, for example, to develop trifluoroacrylic acid obtaining method out of 2,2,3,3-tetrafluoropropanol [80].
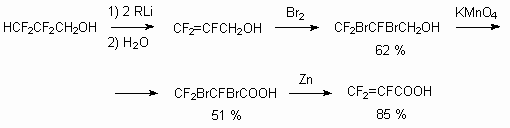
Partly fluorinated alcohols with -CH2CH2OH fragment oxidize rather easily at Jones conditions (oxidization of CrO3 in sulphuric acid) [81]. It is important, that in this case the unsaturated acid is obtained, which contains fluorine atom rather active regarding nucleophiles at internal multiply bond. This is used for synthesis of different heterocyclic compounds and tertiary amines. In case of oxidization of alcohols like RFCF2CH2CH2OH (RF = C5F11, C7F15, C9F19) by CrO3 in sulphuric acid the formation of saturated acids RFCF2CH2COOH is possible, which are also used for synthesis of heterocyclic compounds [82a,b].
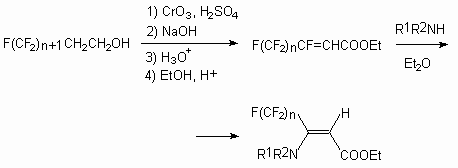
Perfluorinated carboxylic acids F(CF2)nCOOH (n = 1-5) are obtained with high yield and selectivity at acting on partly fluorinated fluoroparafines F(CF2)nCH3 of chlorine or other oxidizing agent at UV-irradiation (2000-14000-A light) [82c].
Analogously, [83,84] unsaturated alcohol (Z+E) is obtained out of H(CF2CF2)5CH2OH by BuLi acting, which is oxidized using ozone in CF3CH2OH medium up to corresponding acid.
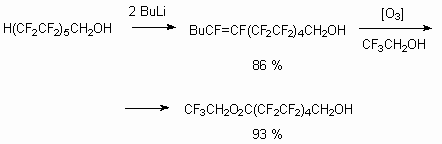
The opportunity of the terminal multiply bond generation can be also used in other purposes.
to be continued
Fluorine Notes, 2004, 35, 1-2
