Fluorine Notes, 2004, 35, 3-4
Perfluoroalkylsulphonyl halides, there synthesis and application
E. Bispen, A..Ilyin, D.D.Moldavsky, S. Nurgalieva
The development of industry is tightly connected with success of chemistry and chemical industry. New chemical compounds, semi-products and composition materials opened wide perspectives nuclear and space industry, medicine and agriculture, electrical engineering and electronics.
In the structure of chemical science and techniques fluorine and fluorine compounds chemistry has a special place. Scarcely becoming familiar with working fluorine methods, mankind had received a great number of unique chemical methods ranging from freons and monomers to components of blood substitutes and perfusion liquids. The reason of perfluorinated compounds wide use in medicine and food industry is their low toxicity, which can be explained by strength of C-F (435-506 kJ/mol)[1,3] bound, its small-scale length, low polarity and shadow effect of shell consisting of fluorine atoms. [8,9,20].
The demand for perfluororganic compounds is constantly growing and it is defined by unique number of chemical, dielectric and thermal and physical characteristics and also by such mutually exclusive characteristics as hydrophoby and moisture permeability.
Perfluororganic compounds, not containing functional groups, have rather wide application and stable demand, but introducing of one or another functional group into organic molecule greatly widens the field of application of compound obtained.
Perfluoroalkylsulphonyl halides (further PFSH ) are used as intermediate compounds for synthesis of surfactants [10-15], lubricants, hydraulic liquids [20,23], ion-exchange membranes and polymer materials 23,24].
In recent years the lowest homologue of this class had passed the tests successfully at obtaining chemical power supplies[20,22] and in laser technics [18]. Thus bis(Perfluoroalkylsulphonyl )imides, obtained out of PFSH are of interest of as Lewis acids [18,19,24] in organic synthesis. Amides with formula: (CF3SO2)2NH, CF3SO2NHSO2(CF2)3CF3 and others are used for obtaining of intercalated compounds like CxN(SO2CF3)2 [18,20]. Salts, obtained on the base of PFSH give an opportunity to use batteries, condensers, supercondensers and galvanic elements at high temperature conditions (up to 350oC) [1,32]. Besides this, the scheme of chemical power supply cationoid part generation is simplified [18,21].
However, the high cost of PFSH , stipulated for absence of efficient obtaining technology, prevents their wide application.
During the process of searching for PFSH obtaining technology the different directions of synthesis both chemical and electrochemical were worked over depending on cost and availability of feed stock, its structure and characteristics of intermediate compounds, including simplicity and safety of one or another obtaining method.
Table 1. Methods synthesis of PFSH (from literature)
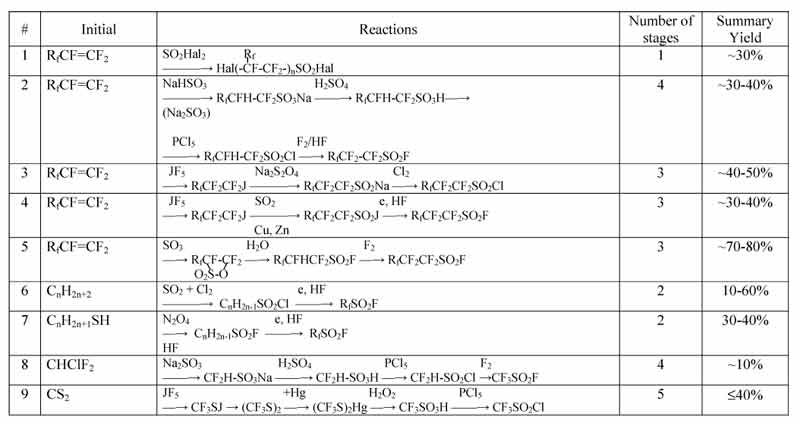
In table 1 the possible options of PFSH synthesis are listed, the number main stages of process and probable yield according to the amount of these stages.
Choosing the raw material, many researchers opted for perfluorolefines, which react easily in reaction of nucleophilic addition. In order to turn tetrafluoroethylene into corresponding biradical 167 kJ/g-mol is required [2].
In a number of works [25-28] the opportunity of PFSH synthesis out of perfluoroolefines and haloid sulphuryl is showed according to the reaction 1:
|
(1) |
where Rf are fluorine, perfluoroalkyl with number of carbon atoms ranging from 1 to 8; Hal - fluorine and/or chlorine.
Sulphuryl fluoride [25], as well as sulphuryl fluorochloride [26] used for this synthesis are reaction-active compounds, which easily interact with
terminal multiply bounds in the molecule of perfluoroolefine.
As a result of reaction (1) the mixture of telomerization products with different number of monomer
chain links and close physical and chemical characteristics [25] is formed as a rule, that impedes isolation of target products and requires using of
complicated rectification. At distillation of reaction mass usually several fractions with wide range of boiling points depending on telomerization rate are obtained. At that, a small number of
chain links (from 5 to 50) produces waxy compounds, which are used as lubricating and surface active [26]. A greater number of
chain links (from 50 to 150) produces powders, which are used for preparation of impregnation compositions, ion-exchange membranes, film materials [25-28].
Another direction of PFSH synthesis using pefluorolefines can be defined as bisulphate method, which was studied in details in the works [29-30]. According to reaction (2)
|
(2) |
monohydroperfluorinated sulphoacid sodium salt is formed. Hydrogen atom is in
![]() position regarding sulphonyl group. The process
goes at temperature up to 120oC [29] in the presence of benzoyl peroxide during the period ranging from 16 to 20 hours. The salt yield is 60-73%. The pressure in autoclave is not controlled and some exceeding of reaction mixture critical points may occur, that can result in explosion. The salt obtained is not a target product
a number of turnings must be carried out to synthesize PFSH :
position regarding sulphonyl group. The process
goes at temperature up to 120oC [29] in the presence of benzoyl peroxide during the period ranging from 16 to 20 hours. The salt yield is 60-73%. The pressure in autoclave is not controlled and some exceeding of reaction mixture critical points may occur, that can result in explosion. The salt obtained is not a target product
a number of turnings must be carried out to synthesize PFSH :
|
(3) |
In the work [31] the opportunity of difluoroacetic acid sulphonyl fluoride obtaining out of chlorodifluormethane and sulphite of alkaline metal is showed, but at that the yield makes up 30-35%.
Perfluoroalkyliodides and perfluoroalkylbromides generating perfluoroalkyl radicals in the presence of initiators were used in a number of works [32-36] along with perfluoroolefines to obtain PFSH . The development of this approach is inseparably linked with the works of Chinese researchers regarding sulphinatodehalogenation of halogen containing hydrocarbons [35-36]. Using Na2S2O4 (sodium dithionite) we can obtain perfluoroalkylsulphinic acid salts out of perfluoroalkylhalogenides with common formula RfX (where X = Br, J) in rather mild conditions. Bromine and iodine, being in perfluorinated carbon chain, are easily substituted and target product is formed with the yield up to 80% [33, 35, 36] according to the reaction (4):
![]()
The obtained sodium perfluoroalkylsulphinate is chlorinated by elementary chlorine to perfluoroalkylsulphonylchloride.
Dithionite method is of absolute interest of science due to transformation of
simple substituents into complicated functional groups, but even in laboratory variant a multistage obtaining scheme is formed, it includes stages of extracting, drying, concentration by evaporation and regeneration. Among with drawbacks of this method we should take in to account:
1) Perfluoroalkyliodides are expensive and scarce products; their obtaining [45-47] requires rather difficult apparatus get-up; parent materials: iodine, iodine pentafluoride and fluorine cause active corrosion, and the production itself is explosive (the reasons of explosion are not studied enough).
2) Now sodium dithionite is not produced in our country; it is not stable at transportation and storage, leads to rising of expenses indexes and decreasing of yield at key stage for 20% and more.
3) 1,5 MT of solid waste products in the form of sodium salts mixture (NaCl, Na2SO4,
Na2S2O3, NaJ) and 2,5 MT of liquid waste products in the form of organic solvents, which regeneration requires additional technological equipment, are formed for 1 ton of target product.
In the work [33] the authors inform, that PFSH are obtained by direct interaction of perfluoroalkyliodides and sulphur dioxide in the presence of zinc-cuprum pair, dispersed in solvent, with further electrochemical fluorination of reaction products (table 1 article 4). However, they speak about laboratory reserach, the yield is not mentioned.
The introducing of sulpohonyl group into organic molecule may be carried out by oxidizing of corresponding disulphides and thiols [1, 37, 48]. Thus in the work [38] thiols are oxidized using nitrogen oxide N2O4, in the medium of hydrogen fluoride according to the reaction (5):
|
(5) |
Where R is alkyl radical, i.e. the initial molecule is not oxidized enough and probably that's why such reaction goes at mild conditions and with the yield of 87%. However, perfluoroalkyl mercaptanes are not discussed in the work.
In the work [1] the trifluoromethylsulphides oxidization using hydrogen peroxide is described, it goes according to the reaction (6):
|
(6) |
with formation of trifluoromethylsulphonic acid.
The obtaining of sulphide consists of several studies (7):
|
(7) |
According to the works [39-48] the introduction of sulphonyl group may be carried out at interaction of pefluoroolefines and
sulphur anhydride with further hydrolysis of sultones obtained (table. 1, article 5). It is known, that the reaction
between perfluoroolefine and distilled sulphur anhydride (![]() -modification), taken in equimolar proportion result in formation of
four-membered cyclic sultones [40]. The reaction goes in autoclave under autonomous pressure, which arises during synthesis at heating-up. This process is rather well studied and sultone is formed with quantitative yield at
moderate temperatures according to the reaction (8)
-modification), taken in equimolar proportion result in formation of
four-membered cyclic sultones [40]. The reaction goes in autoclave under autonomous pressure, which arises during synthesis at heating-up. This process is rather well studied and sultone is formed with quantitative yield at
moderate temperatures according to the reaction (8)
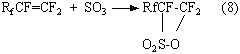
As a result of electrophilic sulphur trioxide attack of double bond
Zwitter-ions is formed (9) [40]
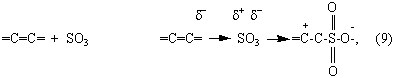
Which later can transform in 4 directions:
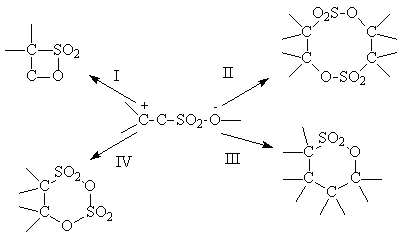
However, according to the information [39, 40] the share of addition products II-IV is very small,
that's why at optimal conditions of synthesis compound I is the main one.
Forming sultones with common formula 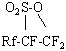 are extremely reactive compounds and interact actively with different mineral and organic compounds.
are extremely reactive compounds and interact actively with different mineral and organic compounds.
Thus, at alkaline hydrolysis of tetrafluoroethan- ![]() -sultone [41] four equivalents of alkali are spent according to the reaction (11)
-sultone [41] four equivalents of alkali are spent according to the reaction (11)
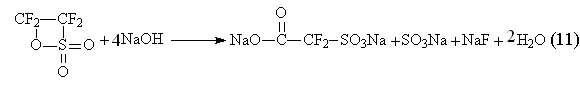
But
![]() - sultones hydrolysis with formation of
- sultones hydrolysis with formation of
![]() - hydropolyfluoroalkylsulзрofluorides is of interest of the working out of PFSH
synthesis method, this hydrolysis goes according to the reaction (12)
- hydropolyfluoroalkylsulзрofluorides is of interest of the working out of PFSH
synthesis method, this hydrolysis goes according to the reaction (12)
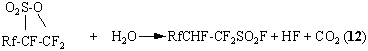
Along with this reaction the concurrent processes are going [40, 42], connected with sultones acid hydrolysis according to the reaction (13)

That lowers the yield of target product for 10-20%.
Hydrolysis reaction of 2![]() -sultones passes with formation of intermediate compound with common formula
-sultones passes with formation of intermediate compound with common formula
![]() .
.
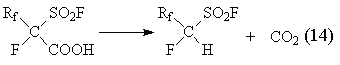
Among these compounds only difluoroacetic acid sulphonyl fluoride is stable [43, 44]. Other compounds of this series
decarboxylize spontaneously forming ![]() -hydropolyfluoroalkylsulphonyl
fluorides according to the reaction (14)
-hydropolyfluoroalkylsulphonyl
fluorides according to the reaction (14)
The obtaining of difluoromethylsulphonyl fluoride is described in the works [24, 49], but suggested methods are not suitable for industrial using.
Higher sultones and ![]() -sultones, obtained on the base of
-sultones, obtained on the base of
![]() ,
, ![]() -diene
perfluoroolefines are easily hydrolyzed with further spontaneous decarboxylizing and formation of corresponding
monohydroperfluoroalkylsulponyl fluorides [48]. These compounds can be considered as relatively available raw material to obtain perfluorinated
alkylsulphonyl fluorides according to the reaction (15)
-diene
perfluoroolefines are easily hydrolyzed with further spontaneous decarboxylizing and formation of corresponding
monohydroperfluoroalkylsulponyl fluorides [48]. These compounds can be considered as relatively available raw material to obtain perfluorinated
alkylsulphonyl fluorides according to the reaction (15)
![]()
However it is known [56], that some attempts to fluorinate cyclic sultones with common formula
![]() were made,
were made,
where Z -1-4 alkylene or chlorosubstituted alkylene or C1-C8 alkyl, OH, Cl, or alkoxygroup. The fluorination was carried out using electrochemical fluorination method in the medium of anhydrous hydrogen fluoride obtaining fluoroanhydrides
that is fluorosulphonylhalogenaliphatic acids with the yield up to 63%.
The method of electrochemical fluorination, developed by Simons [3, 55 - 59] is widely used to obtain perfluorinated compounds of different classes [55 - 59] consists in electrolysis of raw substrate, that's why the success of synthesis is often defined by solubility of raw compound in HF.
Organic compounds of hexavalent sulfur, including aromatic and aliphatic sulphate acids, alkyl and
arylsulphohalogenides (Hal= Cl, Br, J), and cyclic sultones are fluorinated by electrochemical method up
to corresponding perfluoroalkylsulphonyl fluorides. All compounds dissolve rather well in anhydrous hydrogen fluoride, forming current conducting solvents [3, 7, 9].
Now alkylsulfonylhalogenides (chlorides) with common formula CnH2n+1SO2Cl, are used as raw material for PFSH
production, they are obtained according to the Ride reaction (16)
![]()
Usually, during sulphochlorination [4, 23] the chlorination of carbon chain passes according to the reaction (17)
![]()
But at UV-radiation this concurrent reaction is suppressed. Other necessary conditions of this reaction are the presence of initiator and temperature close to room. Nevertheless, along with target products up to 15% of by-products, possessing close physico-chemical characteristics are formed, that requires creation of additional distillation procedures.
To be continued
Fluorine Notes, 2004, 35, 3-4
