Fluorine Notes, 2004, 34, 1-2
Perfluorinated Carboxylic Acids. Synthesis and Application.
Novosibirsk Organic Chemistry Institute of named after N.N. Vorojtsov Of RAS Siberian Department
Ac. Lavrentiev avenue, 9. Novosibirsk, Russia, 630090
E-mail : furin@nioch.nsc.ru
Dedicated to Professor Hermann-Josef Frohn on the Occasion of his 60th Birthday
In this article you will find data regarding perfluorinated carboxylic acids obtaining methods, in particular electrochemical fluorination of hydrocarbon derivatives in anhydrous hydrogen fluoride, perfluoroolefines and telomeric alcohols oxidizing, perfluoralkyliodides and carbon dioxide interaction in the presence of initiators. Some characteristics of perfluorinated carboxylic acids are discussed in this article. Using of these characteristics for creating of new semi-products, used for fluororganic synthesis is discussed here. Practical application examples of both perfluorinated carboxylic acids and semi-products based on them for creating of new generation fluorine materials are given in this article.
Table
of content
1. Introduction. The role of fluorine compounds in development and perfection of materials for the new techniques.
2. The Synthesis of Perfluorinated carboxylic acids.
2.1. Electrochemical fluorination of carboxylic acids and some of their derivatives in anhydrous hydrogen fluoride.
2.2. Oxidization processes of linear and cyclic perfluorolefines and other fluorine-containing
compounds.
2.2.1. The perfluorolefines reactions with ozone.
2.2.2.
Innovations in the oxidization processes of perfluorolefines' double bond up to perfluoroalkanecarboxylic
acids.
2.2.3. The oxidization of telomeric alcohols and polyfluoroaromatic compounds.
2.2.4. The reactions of perfluoroalkylhalogenides under influence of initiators.
2.2.5. The synthesis of perfluorocarboxylic acids out of other classes compounds.
3. The characteristics of perfluorocarboxylic acids and practical application of fluorine materials obtained
on their basis.
3.1. Decarboxylation and decarbonization of perfluorocarboxylic
acids.
3.2. Surface-active materials on the base of perfluorocarboxylic acids derivatives.
3.3. Surface-active agents as fire-extinguishing materials.
3.4. Compositions
for treatment of articles surfaces, rust-proofing coatings.
Conclusion
References.
1. Introduction.
The role of fluorine compounds in development and perfection of materials for the new techniques.
The sweeping development of fluoroorganic compounds chemistry is tightly connected with new materials consumers. They began to be used for atmospheric and salt corrosion protection of metals and alloys and they also started to be used as lubricating oils, used on aggressive conditions, as heat transfers, as liquids for vacuum pumps, working in corrosion active media, as additives to oils, used in compressors of different purposes at increased pressure and they are used in hydraulic liquids.
These perfluorinated semi-products serve as the basis for creation of fluorine materials, which in the future can be used as:
1. Components of flotation reagents for isolation of different non-ferrous metals and mediums at materials and ores mechanochemical desintegration carrying out
2. Lubricants, functioning at high and low temperatures (below -70 oC), increasing durability and mechanical facilities life
3. High temperature heat transfers both for items heating and for heat isolation and utilization
4. Excellent dielectrics and thermal properties allow to use these compounds for items, requiring high security and durability, and they increase fire safety and exploitation of electric machines
5. Fire-safe liquids for oil-filled transformator' leading-ins and oil rectifiers
Perfluorinated compounds are the compounds of present and future. The production of such perfluorinated organic compounds, which meet modern requirements by their thermal and dielectric characteristics, work in a wide range of temperatures and thermal loads and find their practical application, is already settled. The complete substitution of hydrogen atoms by fluorine in the organic molecule results in abrupt changing of properties, that is used to create materials of a new generation with excellent operating characteristics. All this adds such qualities to the materials as:
- incombustibility, un-toxicity,
- low viscosity, low freezing points,
- inertness to metals and
other materials of construction,
- low dissolubility in water and low dissolubility of water
in perfluorinated liquids.
The chemistry and technology of fluoroorganic compounds are the ones of the most fast developing branches of chemistry, that is because of national economy demands in different fluorine materials. Polyfluorinated carboxylic acids should be pointed out of commercially available functional fluorine derivatives [1]. The creating of perspective semi-products, allowing producing different derivatives with polyfluoroalkyl fragment and new fluorine materials on their basis, is an urgent problem, which synthetic chemistry of fluoroorganic compounds is facing.
At every stage of technical progress the role and tendency of fundamental researches, especially the
ones regarding new class organic compounds, is to a great extent initiated by society needs in new
materials, which can't exist without creating fundamentally new materials possessing higher consumer
properties and materials, which can work in more severe conditions. The fluorine compounds exactly
meet such requirements and they can play an essential part for intensification and simplification
of metals production without great reorganization of productions, which already exist. Fluorine chemistry
is a strong independent scientific and technical direction of organic chemistry regarding range and
level of scientific achievements and scale of their production implementation. The proper placing
of priorities and selection of key base semi-products as a base for production of new materials,
which are able to solve principal metal production tasks, are the most important goals. The main
reserve of successful future company work is to apply achievements and projects of scientists well
and to keep with up-to-date demands.
In this review the most of attention is paid to synthesis
and practical application of perfluorinated carboxylic acids and semi-products on their base. Perfluorocarboxylic
acids are exceptionally important multilateral semi-products for synthesis of different fluorine
containing materials. On polyfluorinated carboxylic acids base different salts are obtained, these
salts are used:
- As foaming and fire-fighting agents, as foam stabilizers for production of polyurethane foam, for obtaining of cationic, anionic and non-ionic fluorinated surfactants
- In electrometallurgy and electroplating for metals electrolytic refining
- For creation of effective detergents
- For making materials water and oil- repellent, that is used in different fields of textile, paper and tanning industries. For example, the modification of glyceryl methacrylate using perfluoropolyether derivatives allows to increase polymerization activity and decreasing of solutions surface tension
- As anti-corrosion and insulating metal coatings
- For creating new types of extractive solvent and modifying agent
- High temperature lubricants and coatings of moving details, as wear preventive additives for lubricating oils. The wear of motor oils at introduction only 0.2-2% of additives is 2/3-1/2 of corresponding value for oil, which doesn't contain the additive.
The industrial perfluorinated carboxylic acids obtaining method is based on electrochemical fluorination of carboxylic acids and their derivatives in anhydrous hydrogen fluoride using nickel electrodes [2,3]. Along with that the approaches, where the key stage is the oxidization of different fluorine-containing organic compounds both unsaturated and saturated are developed. In the present review you will find only separate examples of such reactions, at that the main attention was paid to oxidization of perfluorolefines and their derivatives. At the same time it was not an aim to give a full and comprehensive review of perfluorinated organic compounds chemistry, but there had been made an attempt to analyze the main tendencies in development of perfluorinated carboxylic acids synthesis methods for the purpose of revealing new approaches for their obtaining, for showing the opportunity to use perfluoroolefines and perfluoroalkyliodides as initial species for production of such classes compounds.
2. Synthesis of Perfluorinated Carboxylic Acids
2.1. Electrochemical Fluorination of Carboxylic Acids and Some of Their Derivatives in Anhydrous Hydrogen Fluoride.
Perfluorinated carboxylic acids are the most important semi-products and components of fluoroorganic compounds chemistry, that is expressed in great field of their practical application [4-9]. Just the working out of this class compounds synthesis methods became a stimulus at the first stage of electrochemical fluorination method development.
Different compounds, containing C=O group, were introduced into electrochemical fluorination process. Thus, electrochemical fluorination of aldehydes results in formation of fluoroanhydrydes of corresponding perfluorinated carboxylic acids [10].

The use of carboxylic acids themselves at electrolysis in anhydrous hydrogen fluoride results in their total decomposition, the reaction products are partly or totally fluorinated parafines, while dicarboxylic acids and their derivatives are being fluorinated smoothly, but intramolecular cyclization takes place, which results in formation of penta- and hexa-component heterocyclic compounds [11,12]. It is interesting, that the presence of two groups - SO2F and COF in the molecule is accompanied by cyclization along with affecting of COF group and forming of fluoroanhydride of corresponding sulpho-acid.

The formation of cyclic esters at electrochemical fluorination of dibasic acid derivatives was an undesirable process, because it lowered the yield of commercial carboxylic acids. However, the fields of application were found for them later. Now they are considered as most perspective compounds for compositions of artificial blood and for storage of transplantation organs, for dielectrics and coolers for wide class of transformers, high-temperature heat transfers. That's why the interest to perfluorinated cyclic esters synthesis according to this method doesn't surprise, because direct fluorination method is limited due to low availability of source hydrocarbons that is cyclic ethers.
In case of electrochemical fluorination of dibasic acid chloroanhydrides [13,14] and lactones [15] fluoroanhydrides of dicarboxylic acids are formed.

The formation of new heterocyclic compounds is observed at electrochemical fluorination of dicarboxylic acid ethyl esters, that takes place due to intramolecular cyclization, passing with the participation of oxygen atom C=O group. Analogously perfluorodioxolan is obtained out of EtO2C(CF2)nCO2Et (n = 1-6) [16] also.

Contrary to this, the yield of perfluorocarboxylic acid fluoroanhydrides greatly increases and becomes defining product, if using as source substrate not the acids themselves, but fluoroanhydrides or chloroanhydrides of corresponding acids (table. 1) [17,18].

Table 1. Electrochemical fluorination of carboxylic acid derivatives [22,23].
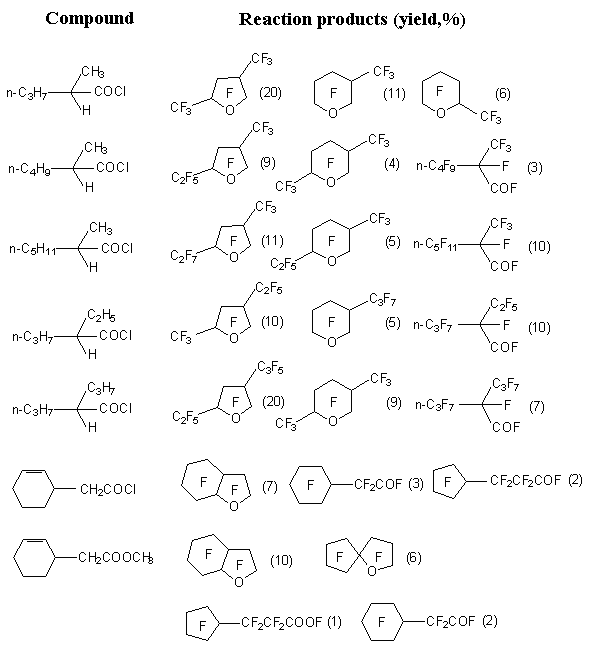
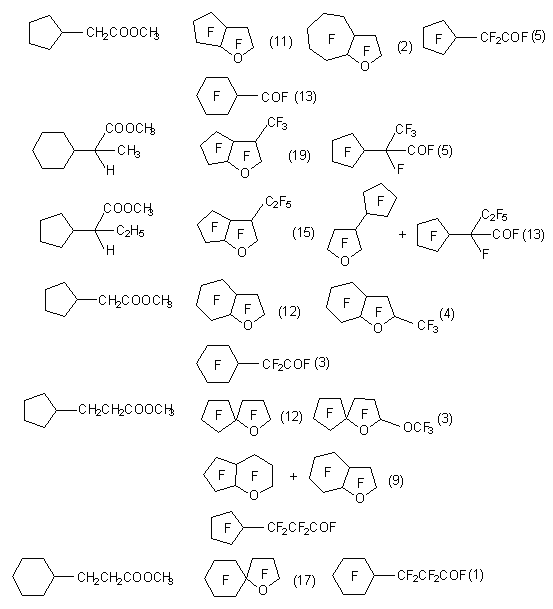
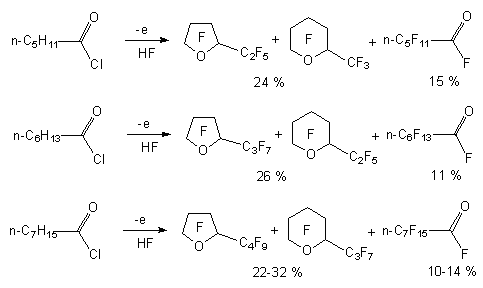
At electrochemical fluorination of acetic acid fluoroanhydride the trifluoroacetate fluoride is obtained, it's yield is 36-45% (according to current the yield is 32-50%) [17,19,20]. The electrochemical fluorination of carboxylic acid chloroanhydrides, for example, chloroanhydrides of caproic, enanthic, caprylic and capric acids results in formation of fluoroanhydrides of corresponding perfluorinated acids with small yield (11-15%), the main products of this process are perfluorinated cyclic ethers (24-35 %) [5].
Esters of carboxylic acids at electrochemical fluorination in anhydrous hydrogen fluoride usually saponify and in the beginning fluoranhydrides of corresponding acids are formed, which further are subject to fluorination. However, perfluorinated heterocyclic compounds are obtained in this case too. [13,21].
Detailed studying of this process showed, that substrates, containing cycloalkyl groups as substituents produce bicyclic ethers with satisfactory yields (table 1) [20]. Such cyclic ethers contain two penta- component cycles or one penta-component and one hexa-component. The yield of products is not very high. Besides, in small quantities the corresponding perfluorinated esters are formed out of acids and the products of their transformation - perfluoroparafines and perfluoroacyclic derivatives are formed also [11].
At electrochemical fluorination of methyl
2-cyclohexylacetate and some other derivatives
of carboxylic acids the authors of works
[18,24,25] found new cyclization, passing
with participation of multiply bond,
resulting in formation of heterocyclic
compounds. This process is general for
carbonyl compounds, having multiply bonds
in  -position.
-position.
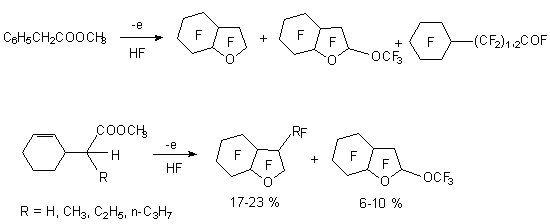
Perfluoro(alkoxycycloalkane) derivatives of carbonyl fluoride
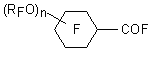
where R is perfluoroalkyl C1-C4;
n = 2-5, is obtained by electrochemical fluorination of corresponding acid chloroanhydrides, for example, 3,4,5-(MeO)3C6H2COCl in the presence of 10% dimethylsulphide in boiling anhydrous hydrogen fluoride at 2000 tor and at 50 oC (nickel anode) gave 3,4,5-(CF3O)3C6F8COF [26] with the yield of 30 %. These compounds have surfactants properties and they are used as film forming foams.
Fluorine Notes, 2004, 34, 1-2
