Fluorine Notes, 2004, 33, 1-2
THE SYNTHESIS AND SOME CHARACTERISTICS OF PARTLY FLUORINATED ALCOHOLS ON THE BASIS OF TETRAFLUOROETHYLENE AND HEXAFLUOROPROPYLENE
(REVIEW)
G.G. Furin
The Organic Chemistry Institute named after N.N. Vorojtzov of the Novosibirsk city Sibirian department of Russian Academy of Sciences
9, Academic Ak. Lavrentiev avenue, Novosibirsk, 6300090 Russia
E-mail :furin@nioch.nsc.ru
In this review the approaches of partly fluorinated alcohols on the tetrafluoroethylene and hexafluoropropylene basis are analyzed. New experimental data regarding telomeric alcohols involvement into reactions with unsaturated compounds and hetero-organic derivatives are discussed. Oxidization processes and reactions in strong acid mediums of telomeric alcohols are analyzed. Also in this review you will find the trends of practical using of telomeric alcohols. The questions regarding partly fluorinated alcohols toxicity and some of their derivatives are discussed here.
Table of contents
Introduction. The role of fluorine containing compounds in new fluorine materials creation.
1. The development of partly fluorinated alcohols obtaining technology using interaction of teterafluoroethylene and hexafluoropropylene with alcohols in the presence of radical initiators.
2. Partly fluorinated alcohols as effective O-nucleophilic reagents, their application for creating of fluorine containing semi-products and materials on their basis.
2.1. The interaction of trimethylsilyl ethers of partly fluorinated alcohols with unsaturated compounds – the synthesis way of wide application field dialkyl ethers.
2.2. The reactions of polyfluoroaromatic compounds with telomeric alcohols and creating of new materials on their basis.
2.3. The interaction of telomeric alcohols with hetero-organic compounds.
2.4. The oxidization of telomeric alcohols till polyfluorinated carboxylic acids and their (acids) practical use.
2.5. Dielectric heat-transfers synthesis on the base of partly fluorinated alcohols.
2.6. The processes with telomeric alcohols participation.
3. The toxicity of partly fluorinated alcohols and some derivatives.
References
2.4. The oxidization of telomeric alcohols down to polyfluorinated carbone acids and practical use of these acids.
Telomeric alcohols (n = 4, 5) can be used as ingredient of surfactant compositions. Thus the addition of telomeric alcohol (n=4) to sodium laurylsulphate provides 1,5 time surface tension lowering compare to of pure sodium laurylsulphate. It can be used for metal electrolytic refining and ore flotation.
Telomeric alcohols can be oxidized till corresponding 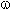 -hydroperfluorocarboxylic acids, which are important for creation of different fluorine materials for practical purpose, among which we'll point out surface active materials, which are used as emulsifiers at polymerization of fluorolefines, as basis for universal foaming agent, work liquids, oil dopes, pesticides, bactericidal and pharmaceutical compositions.
-hydroperfluorocarboxylic acids, which are important for creation of different fluorine materials for practical purpose, among which we'll point out surface active materials, which are used as emulsifiers at polymerization of fluorolefines, as basis for universal foaming agent, work liquids, oil dopes, pesticides, bactericidal and pharmaceutical compositions.
Different salts are obtained on the base of polyfluorinated carboxylic acids. They are used:
- As foaming agents and products for fire extinguishing, as foam stabilizers for production of polyurethane foam,
- In electrometallurgy and electroplating for metals' refining,
- for creation of effective cleansers,
- for materials' treatment and making them water-repellent and oil-repellent ,
- as slushing and insulating metal coatings,
- for creation of new types of extragents and metal dressers,
- for creation of high-temperature lubricants and moving details coatings, they are used as wear-preventive additives for lubricating oils.
The deterioration of motor oils at 0.2-2% admixture's addition is 2/3 -1/2 of corresponding value for the oil, which doesn't contain the admixture.
Poly-fluorinated carboxylic acids H(CF2CF2)nCOOH (n = 1-5) can be semi-products for obtaining of terminal poly-fluorolefines.
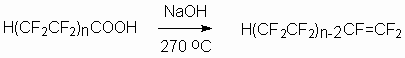
The last ones mentioned can be used for:
- obtaining of polyfluoroalkansulpho acids and obtaining on their basis of surface active materials and electrolytes for lithium batteries and rechargeable accumulators,
- creating of new complex-formers for rare elements' salts,
- production of high-temperature liquid dielectrics, heat-transfers and hydraulic liquids.
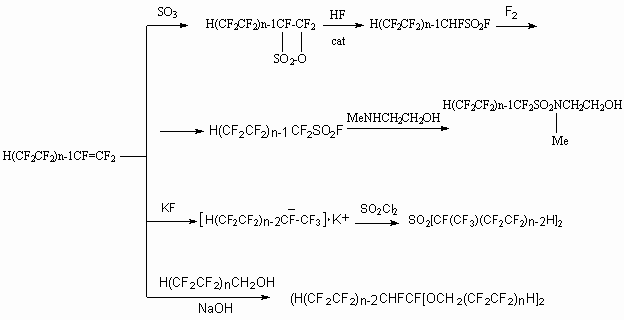
The oxidization of telomeric alcohols is carried out using potassium permanganate action, nitrogen oxides, chrome anhydride, chlorine at 100-140 oC and UV irradiation.
However these methods have essential disadvantages and manufacturing technologies are not developed on their basis. Thereby the oxidization processes of telomeric alcohols are still in the focus of researchers.
Thus, telomeric alcohols (C1-16) are oxidized using nitric acid in the presence of catalysts, which are oxides and salts of ferrum, nickel, copper and vanadium [109]. At oxidization of H(CF2)8CH2OH by 55 %-nitric acid and simultaneous oxygen supply at 125oC and P=0,8 MPa in the presence of FeCl2 *nH2O after 7 hours at 100 % conversion of alcohol the corresponding acid H(CF2)8COOH is obtained with 99.6 % yield [109].
Higher carbon acids are obtained using electrochemical fluorination of telomeric alcohols H(CF2CF2)nCH2OH , (n = 2-5) in anhydrous hydrogen fluoride [27]. The last have found their application at emulsion polymerization of tetrfluoroethylene.
Catalytic system, containing bivalent copper complexes, catalyzes selective oxidization of telomeric alcohols till perfluorocarboxylic acids [110]. This method is especially effective for alcohols with long hydrocarbon chain (C8-14). The process passes in alkaline medium, which is necessary for alcohol conversion into anion form, that essentially facilitates the molecular oxygen activation at low temperatures.
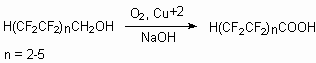
The participation of copper complexes in the transfer of electrons from alcoholates ions to oxygen obviously allows the reaction to pass according to thermodynamically efficient di-electrone mechanism, that determines for abnormally high oxidization rates. The rate of reaction doesn't depend on molecular mass of oxidized alcohols in the interval n = 1-6. Variation of reaction carrying out conditions (temperature, solvent, ligand, alkali and catalyst concentration and so on) allows oxidizing of telomeric alcohols selectively (100%). For example, HCF2CF2CH2OH is oxidized by molecular oxygen in the presence of copper catalyst and alkali agent (in the presence of 5*103 M solution CuCl2 and 1.10-2 M solution of ortho-phenanthroline in iso-butyl alcohol and NaOH at 45 oC, 6.5 hours) with the yield of 73.8 % to HCF2CF2COOH (conversion = 50.5 %).
Oxidization of telomeric alcohols H(CF2)nCH2OH (n = 2, 4, 6) in the liquid phase by air oxygen at 350 oC in the presence of V2O5 results in formation of H(CF2)nCH(OH)2 with the yield 74-93 % [111].
The authors [112] worked out the approach to 3- chlorotetrafluoropropionic acid synthesis using photochemical chlorination of telomeric alcohol HCF2CF2CH2OH borate and phosphate esters.
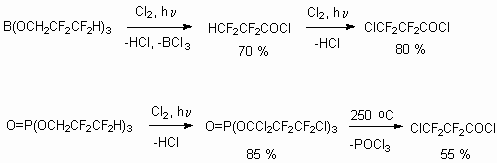
The chlorine action is processed at UV-radiation (lamp of 100-400 W) at 25-110 oC, at that consecutive replacement of  - and
- and  - hydrogen atoms of alcohol can occur. Decomposition of chlorinated borate passes now at conditions of reaction till acid chlorine anhydride is formed, while chlorinated phosphates require high temperatures (180-250 oC). At the same time the telomeric alcohols' effect of chlorine in the presence of catalyst results in formation of 1-chloroperfluoroalkanes [113].
- hydrogen atoms of alcohol can occur. Decomposition of chlorinated borate passes now at conditions of reaction till acid chlorine anhydride is formed, while chlorinated phosphates require high temperatures (180-250 oC). At the same time the telomeric alcohols' effect of chlorine in the presence of catalyst results in formation of 1-chloroperfluoroalkanes [113].
Telomeric alcohols can act as intermediate products to obtain high-temperature and high efferctive heat transfers [114], dielectrics [115], and they can act as effective lubricating oils and compositions, for example at modification of tosyl ethers based on phenols and telomeric alcohols [116]. It is stated, that the formation of corresponding acetals [117,118] occurs during reaction of telomeric alcohols with formaldehyde in concentrated sulfuric acid.
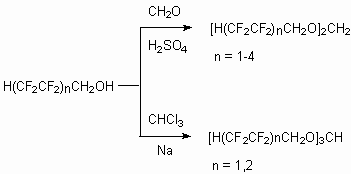
Fluoroalkyl ortho ethers [119] are obtained at interaction with chloroform in the presence of alkali metals. These ethers are thermal resistant and their liquid boiling points are high, so they can find their application as heat transfers, while their additional fluorination using element fluorine results in formation of perfluorinated ethers, which be of interest of as liquid dielectrics.
2.6. The processes with telomeric alcohols.
Telomeric alcohols have two reaction centers, according to which the reactions can be carried out. Above we had discussed the telomeric alcohols reactivity in reactions, passed affecting alcohol group. At the same time the presence of CHF2 fragment at the end of carbon chain allows to expect the turnings with its active participation. First of all, the presence of fluorine atoms and the influence of CF2fragment being alongside results in acidity increasing and thereafter this makes carbanion simulated emission possible. Indeed, the action of two moles of alkyl lithium leads to formation of terminal multiply bond [26]. This, for example, allows to work out the trifluoroacrylic acid obtaining method using 2,2,3,3-tetrafluoropropyl alcohol as raw product.
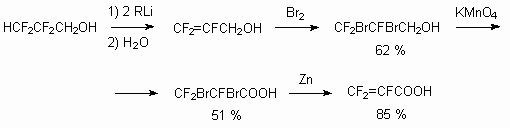
If you affect allyl alcohol with concentrated sulphuric acid, then  -fluoroacrylic acid fluoroanhydride will be formed [120].
-fluoroacrylic acid fluoroanhydride will be formed [120].
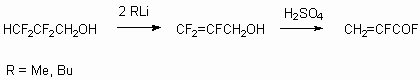
The ozone oxidized unsaturated alcohol (Z+E) [121] in CF3CH2OH medium till corresponding acid is obtained analogously by BuLi action out of H(CF2CF2)5CH2OH.
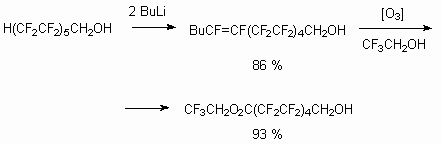
The possibility of terminal multiply bond simulated emission can be used for other purposes also.
3. Toxicity of Partly fluorinated alcohols and some of its derivatives.
There had been carried out the researches of muscles regarding studying of toxicological parameters [122,123] for partly fluorinated dialkyl ethers telomeric alcohols and partly fluorinated carbon acid esters. The results are listed in table 3. Judging by data of this table 3 we can make a conclusion, that studied compounds are referred to III or IV class (Russian classification) of medium or low toxic compounds. It is stated, that dialkyl ethers do not locally affect skin and eye mucous membrane. Telomeric alcohols in sub-toxic dose (1000 mg/kg) bring on the suppression of respiratory center and also central nervous system, appearing as strong sedative effect and movement disorder of mice. These neurotoxic evidences are restored to physiological norm in 24 hours. When you rate the danger level of these compounds for a human being it is necessary to take into account their possible influence on myocardium function, thyroid gland, and also their gonado-and- genotoxic effect. This data allow using these compounds for production of fluoromaterials and for their production technology successfully.
Table 3. Some toxicological parameters of fluorine containing compounds[122,123].
| Compound | LD50 mg/kg | LD100 mg/kg | Dangerous class |
| CF3CHFCF2OCH2CF2CHF2 | 5000 | IV | |
| HCF2CF2CH2OCH2CH3 | 3420 | 6000 | III |
| HCF2CF2CH2OH | 2320 | 3000 | III |
| HCF2CF2CF2CF2CH2OH | 1180 | 2000 | III |
| CF3CHFC(O)OC2H5 | 5000 | IV | |
| HCF2CF2CH2OCHF2 | 5000 | IV | |
| CH2[OCH2(CF2CF2)2H]2 | 5000 | IV | |
| CH2[OCH(CH3)CF2CF2H]2 | 1917 | 5000 | III |
| HCF2CF2C(CH3)=CH2 | 1334 | 3000 | III |
| CF3CHFCF2CH2OH | 640 | 1000 | III |
 | 5000 | IV |
Conclusion
The above material allows to establish the growing researchers' interest in working out of approaches of poly-fluorinated fragments application into organic molecules and approaches of simple substitutes transformation into complicated functional alignments. In this regard the use of partly fluorinated alcohols, obtained on the basis of polyfluorolefines, opens up possibilities for new fluorine-containing materials synthesis. Much success, lately achieved in working out of partly fluorinated alcohols synthesis method, using which (p. f. Alcohols) you are able to carry out the fluorine- containing fragments application into organic molecules, displays that they in a few cases can be an alternative for classical and well-known methods. A number of processes have obvious advantages and real application opportunities in industrial technologies. There is no doubt, that issues, connected with ideas and processes realization, are of interest of both chemists, working in the field of fluoroorganic synthesis and specialists in the field of practical use of fluoromaterials on the base of fluorine organic compounds. In this review the author did his best to demonstrate new approaches and synthetic possibilities of new semi-products basically by example of telomeric alcohols, he also tried to display tendencies and main directions of researches in the field of synthesis of poly-fluoroorganic compounds, which contain different molecular frames and functional groups. Thus, commercialized by industry process of telomeric alcohols obtaining gave a stimulus of their wide and thorough research and synthesis of new fluorine materials, since such processes are rather simple and can be implemented on industrial scale/basis. We can hope, that later there will be revealed other reactions, resulting in fluorine-containing materials.
2. Igumnov C.M. Bazanov A.G.,
Shipigusev A.A.‚ Soshin V.A., Lekontseva G.I., Il'in A.N., Denisenkov V.F. //
Pat. 2134257 RU (1999). 3. Barabanov V.G., Denisenkov V.F., Il'in A.N., Novgorodov V.N., Trukshin
I.G., ~15. 5-8 June. 2001. Helsinki. Finland. Abstracts. PP-9. P. 259. 4. Tereshchenko G.F, Kuznetsov
A.S., Il’inА.N., Denisenkov V.F.,
Uklonskii I.P. // Pat. 2124493 RU (1999). 5. Denisenkov V.F.,
Il`in A.N., Volkov A.F., Lekomtsev A.G., Ivanova L.M., Plotnikov V.F. //Pat.
2074162 RU (1997) 6. Il'in A.N., Ivanova L.M. //
Mezhdunarodnaya nauchno-tekhnicheskaya konferentsiya «Perspektivy himicheskoj
tekhnologii i materialov».Perm', 1997. Tez. dokl, s.203 7.
Yamaguchi F., Takaki S., Yoshizawa T., Ogura E., Katsube T. / US Pat. 6187969 (2001) 8.UklonskiiI.P.,Denisenkov
V.F., Il`in A.N., Ivanova L.M., Bakhmutov Yu.L., Mineev S.N. //Pat. Russia
2150459 (2000); CA 2002. Vol. 136. 218614b. 9. Blickle P.,
Heumueller R., Hintzer K., Schwertfeger W., von Werner K. / Ger. Offen DE
3915759 (1990); CA1991, Vol. 114, 184789r 10. Haszeldine
R.N., Rowland R., Sheppard R.P., Tipping A.E. // J. Fluorine Chem. 1985. Vol.
28. P. 291. 11. Paleta O.,
Dedek V., Routschek H., Timpe H.-J. // J. Fluorine Chem. 1989. Vol. 42. P. 345. 12. Paleta O.,
Dedek V., Neuenfeld S., Timpe H.-J. // Czech. Pat. 268 247 (1989). 13. Takaki S.,
Yoshizawa T. / Eur. Pat. Appl. EP 968990 (2000); CA2000. Vol. 132. 65740e. 14. Yoshizawa T.,
Takaki S. / PCT Int. Appl. WO 02 18308 (2002); CA2002. Vol. 136. 233886k. 15. Yamaguchi F.,
Katsube T. / Pat. 6313357 US (2001) 16. Takaki S.,
Yoshizawa T. / Pat. 6294704 US (2001) 17. Yoshizawa T.,
Takaki S. / PCT Int. Appl. WO 02 18309 (2002); CA2002. Vol. 136. 218630d. 18. Guidot J.,
Alric J., Ameduri B., Rousseau A., Boutevin B. // New J. Chem. 2001. Vol. 25. N
9. P. 1185-1190; CA2002. Vol. 136. 37984b. 19. Chambers R.D.,
Grievson B. // J. Chem. Soc., Perkin Trans, 1, 1985. P. 2215; 20. Chambers R.D.,
Grievson B. // J. Fluorine Chem., 1985. Vol. 29. P. 323; 21. Motherwell
W.B., Crich D., Free Radical Chain Reactions in Organic Synthesis. Academic
Press Ltd. : London. 1992. 22. No V.B.,
Bakhmutov Yu.L., Pospelova N.B. // Izv. Akad. Nauk SSSR. Ser. Khim. 1976.N 8. P. 1825. 23. Rahimov A.I., Vostrikova
O.V., Ermakov A.V. / Tez. dokl.5 mezhdunarodnoj konf. «Naukoemk. Khim.
Tekhnol.»Yaroslavl', 19-21 may 1998, t. 1, s.
141-142. 24.
Skibida I.P., Sakharov A.M., Bakhmutov J.L., Denisenkov V.F., Martynova N.P. / Pat.
US 5495034 (1996) 25.Ichihara K., Aoyama H. / Eur. Pat. Appl. 1085006
(2001) 26. Nguyen T.,
Wakselman C. // Synth. Commun. 1990. Vol. 20. N 1. P. 97-99. 27. Maximov, Kosareva,
Riabinin // Pat. Russia 2107751 (1993) 28. Szlavik Z.,
Tarkanyi G., Skribanek Z., Vass E., Rabai J. // Org. Lett. 2001. Vol. 3. N 15.
P. 2365-2366; CA2001. Vol. 135. 210736b. 29. Wada A.,
Tanaka H., Tanabe K., Yamagishi N., Toma T. / Eur. Pat. Appl.1191009 (2002) 30. Wada A.,
Fujima T., Kawahara K. // Jpn. Kokai Tokkyo Koho JP 2002173454 (2002); CA2002.
Vol. 137. 20153. 31. Yoshizawa T.,
Takaki S., Yasuhara T., Yokoyama Y. / Eur. Pat. Appl.1179521 (2002) 32. Oharu K. / PCT
Int. Appl. WO 2002028807 (2002); CA2002. Vol. 136. 294534r. 33. Ishihara K.,
Homoto Y., Baba N. / Jpn Kokai Tokkyo Koho JP 2002053505 (2002); CA2002. Vol.
136. 167088y. 34. Yoshizawa T.,
Takaki S., Yasuhara T., Yokoyama Y. // Eur. Pat. Appl. EP 967193 (1999);
CA2000. Vol. 132. 51457f. 35. Yamaguchi f.,
Takaki S., Yoshizawa T., Ogura E., Katsube T. / Eur. Past. Appl. EP 968989
(2000); CA2000. Vol. 132. 65739m. 36. Yamaguchi F.,
Katsube T. // Jpn. Kokai Tokkyo Koho JP 2000273060 (2000); CA2000. Vol. 133.
252044. 37. Tohma T., Wada
A. / PCT Int. Appl. WO 2002020444 (2002); CA2002. Vol. 136. 249368d. 38. Yoshizawa T.,
Takaki S. / PCT Int. Appl. WO 2002018307 (2002); CA2002. Vol. 136. 216455b. 39. Yamaguchi F.,
Takaki S., Yoshizawa T., Ichihara K. / PCT Int. Appl. WO 2002018306 (2002);
CA2002. Vol. 136. 216454a. 40. Yoshizawa T.,
Takaki S. / PCT Int. Appl. WO 2002018309 (2002); CA2002. Vol. 136. 218630d. 41. Okamoto H. /
PCT Int. Appl. WO 2002016295 (2002); CA2002. Vol. 136. 199936q. 42. Cirkva V.,
Polak R., Paleta O. // J. Fluorine Chem. 1996. Vol. 80. P. 135-144. 43. Furin G.G.,
Il`in A.A., Suchinin V.S., Tolstikova T.G. // Zh. Prikl. Khim., in press. 44. Guiot J.,
Alric J., Ameduri B., Rousseau A., Boutevin B.// New J. Chem., 2001. Vol. 25. N
9. P. 1185-1190. 45. Paleta O.,
Cirkva V., Kvicala J. // Macromol. Symp. 1994. Vol. 82. P. 111. 46. Kurykin M.A.,
German L.S., Kartasheva L.I., Pikaev A.K. // J. Fluorine Chem. 1996. Vol. 77.
P. 193-194. 47. Chambers R.D.,
Kelly N., Emsley J.M., Jones W.G.M. // J. Fluorine Chem., 1979. Vol. 13. P. 49. 48. Muramatsu H.,
Moriguchi S., Inukai K. // J. Org. Chem., 1966. Vol. 31. P. 1306. 49. Chambers R.D.,
Diter P., Dunn S.N., Farren C., Sandford G., Batsonov A.S., Howard J.A.K. // J.
Chem. Soc., Perkin Trans, 1, 2000. P. 1639-1649. 50. Chambers R.D.,
Hutchinson J., Kelly N.M., Joel A.K., Rees A.J., Telford P.TY. // Isr. J. Chem.
1999. Vol. 39. N 2. P. 133-140; CA1999. Vol. 131. 299128t. 51. Udagawa A. //
Jpn. Kokai Tokkyo Koho JP 240571 (2001); CA2001. Vol. 135. 212588d. 52. Thenappan A.,
Van der Puy M., Eibeck R. // Pat. 5629458 US (1997); CA1997. Vol. 126. 317174. 53. Gillet J.-P.
// PCT Int. Appl. WO 9721656 (1997); CA1997. Vol. 127. 108705. 54. Molchanova
G.N., Petrovskii P.V., Shcherbina T.M., Laretina A.P., Zakharov L.S., Kabachnik
M.I. // Izv. Akad. Nauk. Se. Khim. 1996. N 6. P. 1516-1519; CA1996. Vol. 125.
247923. 55. Miki A.,
Aoyama H. // Jpn. Kokai Tokkyo Koho JP 2000038361 (2000); Chem Abstr. 2000.
Vol. 132. 122303. 56. Arrowsmith
C.H., Kresge A.J., Tang Y.C. // J. Am. Chem. Soc., 1991. Vol. 113. N 1. P.
179-182. 57. Smart B.E. in
Organofluorine Chemistry - Principles and Commercial Applications. / Eds. R.E.
Banks, B.E. Smart, J.C. Tatlow. Plenum Press, London. 1994. Chapter 3. P. 57. 58. Nakahara A., Tono
S. // Jpn. Kokai Tokkyo Koho JP 09309849 (1997); CA1997. Vol. 128. 22630. 59. Chen Q.-Y., Wu
J.-P. // J. Chem. Res.Synop.
1990. P. 268; CA1990. Vol. 113. 211461. 60. Grinevskaya
V.K., Petrov V.A., German L.S. // 1th International Conference «Chemistry,
Technology and Application of Fluorocompounds in Industry», May 30 - June 3,
1994. St. Petersburg. Russia. Abstracts. P4-32. P. 196. 61. Moldavskii
D.D., Furin G.G. // Zh. Prikl. Khim. 1985. Vol. 68. N 12. P. 2018-2022; CA1995.
Vol. 125. 194689. 62. Patel N.R.,
Chen J., Zhang Y.-F., Nishida M., Kirchmeier R.L., Shreeve J.-M. // Inorg.
Chem. 1994. Vol. 33. P. 5463-5470. 63. Chen J.,
Kirchmeier R.L., Shreeve J.M. // Inorg. Chem. 1996. Vol. 35. N 6. P. 1718-1720. 64. Prakash
G.K.S., Krishnamurti R., Olah G.A. // J. Am. Chem. Soc., 1989. Vol. 111. P.
393-395. 65. Farnham W.B.,
Nappa M.J. // Pat. 5457215 US (1995) 66. Elias A.J.,
Kirchmeier R.L., Shreeve J.-M. // 207th ACS National Meeting.
San Diego. USA. 1994. 67. Montanari V.,
Quici S., Resnati G. // Tetrahedron Lett. 1994. Vol. 35. P. 1941-1944. 68. Chi K.-W.,
Furin G.G. // Bull. Korean Chem. Soc., 2000. Vol. 21. N 6. P. 641-643. 69. Boutevin B.,
Youssef B. // J/ Fluorine Chem. 1989. Vol. 44. P. 295. 70. Korobeynicheva
I.K., Andreevskay O.I., Podgornay M.I., Furin G.G. // Izv. Akad. Nauk SSSR.
Ser. Khim. 1982. P. 103. 71. Lehmann L.,
Dvornikova K.V., Platonov V.E., Prescher D. // J. Fluorine Chem. 1991. Vol. 54.
P. 186. 72. Larionov S.V.,
Kosareva L.A. // Izv. SO RAN. Ser.Khem. n., 1989. N 3. P. 83-86. 73.
Patent SU 966971 74.
Otrozhdennova L.A., Ryaboi V.I., Poroshina A.N., Tsarenko S.V., Bil'dinov K.N.
// Patent SU 914088 (1979). 75.
Voronkov M.G., Chernov N.F., Fedorova E.O. . // 1th International Conference
«Chemistry, Technology and Application of Fluorocompounds in Industry», May 30
- June 3, 1994. St. Petersburg. Russia. Abstracts. L2-8. P. 70. 76. Bassilana
Serrurier C., Cambon A. // J. Fluorine Chem. 1998.Vol. 87. N 1. P. 37-40. 77. Boguslavskaya
L.S., Panteleeva I.Yu., Morozova T.V., Monich I.M., Chuvatkin N.N. . // 1th
International Conference «Chemistry, Technology and Application of
Fluorocompounds in Industry», May 30 - June 3, 1994. St. Petersburg. Russia.
Abstracts. P2-12. P. 87. 78. Akira O.,
Shoji T., Takahiro K. // Pat EPO 1366668 (1985). 79. Akira O.,
Shoji T., Takahiro K. // Pat 4604482 US (1987) 80. Akira O.,
Takahiro K. , Shoji T. // Japan Appl. 61-85345 (1987) 81. Akira O.,
Nobuyuki T., Takahiro K. // Pat EPO 128516 (1985); CA1985. Vol. 102. 185964h. 82. Akira O.,
Nobuyuki T., Takahiro K. // Pat EPO 128517 (1985); CA1985.Vol. 102. 115070g. 83. Akira O.,
Takahiro T., Takahiro K. // Japan Appl. 60-118808 (1987) 84. Bosc D.,
Boutevin B., Pietrasanta Y., Rousseau A. // France Appl. 2623510 (1990) 85. Jjaa S., Koji
N., Masoru M., Takashi J. // Japan Appl. 61-121005 (1986); CA1986. Vol. 105. 192481. 86. Takashi J.,
Katsuhiko S., Ryuji M. et al. // Japan Appl. 61-240205 (1987); CA1987.Vol. 106. 139496. 87. Tategami Y.,
Fujita K., Furuta M., Tamura T. // Japan Appl. 60-250309 (1986); CA1986.Vol. 104. 226030n. 88. Joshiharu T.,
Katsuramaru T., Motonobu F., Tashibubnu T. // Japan Appl. 61-208006 (1987);
CA1987.Vol. 106. 68407. 89. Shigeru M., Masahiko O. // Japan Appl. 61-86448 (1986);
CA1986. Vol. 105. 157727. 90. Akiro O.,
Kazuo I. // Pat.EPO 158113
(1985); CA1985. Vol. 104. 89225. 91. Timperley
C.M., Broderick J.F., Holden I., Morton I.J., Waters M.J. // J. Fluorine Chem.,
2000.Vol. 106. N 1. P.
43-52. 92. Timperley
C.M., Arbon R.E., Saunders S.A., Waters M.J. // J. Fluorine Chem., 2002. Vol.
113. N 1. P. 65-78. 93. Markovsky
L.N., Bobkova L.S., Pashinnik V.E. // Zh. Org.Khim. 1981. Vol. 17. N. 9. P. 1903-1908. 94. Markovsky L.N., Bobkova L.S., Pashinnik V.E., Iksanova S.V. //
Zh. Org. Khim. 1981. Vol. 17. N 3. P. 486-490. 95. Pashinnik V.E., Tovstenko V.I.,Bobkova L.S., Markovsky L.N. //
Zh. Org. Khim. 1985. Vol. 21. N. 10. P. 2072-2076. 96. Markovsky L.N., Pashinnik V.E., Tovstenko V.I., Yaremenko V.V.,
Kolesnik N.P., Shermolovich Yu.G. // Zh. Org. Khim. 1990. Vol. 26. N 11. P.
2451-2452. 97. Markovsky L.N., Tovstenko V.I., Pashinnik V.E., Melnichuk
E.A., Makarenko A.G., Shermolovich Yu.G. // Zh. Org. Khim. 1991. Vol. 27. N 4.
P. 769-773. 98. Pashinnik V.E.
// Ukr. Khim Zh. 1999.Vol.
65. N 11. P. 17-20. 99.
Efanova E. Yu. // Avtoreferat dissertatsii na soiskanie k.kh.n., 2002,
Volgograd. 100.
Storojakova N.A., Fedunov R.G., Rakhimov A.I., Efanova E.Yu. // Zh.Phys. Khem. 2002. Vol. 76. P. 2260-2263. 101. Storozhakova
N.A., Zheltobryukov V.F., Rakhimov A.I., Zauer E.A. // The 3d International
Ñonference «Chemistry, Technology and Applications of Fluorocompounds”, CTAF`
2001. June 6-9, 2001. St. Petersburg. Russia. Abstracts. P2-9. P. 74. 102. Berkessel A.,
Anddreae M.R. // Ger.Offen.
DE 10055653 (2002); CA2002. Vol. 136. 355170. 103. Mantani T.,
Shiomi K., Konno T., Ishihara T., Yamanaka H. // J. Org.Chem., 2001. Vol. 66. P. 3442-3448. 104.
Patent SU 1223595, Patent SU 1148285, Patent SU 1272670 105. Millauer H.
// Ger. Offen 3128118 ÔÐÃ (1983); CA1983.Vol. 98. 178738h. 106.
Rahimov A.I., Ermakov A.V. // Khimiya i tekhnologiya ehlementoorganicheskih
monomerov i polimernyh materialov. Izd. Volgogradskogo gos. Tekhnich.
Universiteta. Volgograd, 1997. 107.
Rakhimov A.I., Vostrikova O.V. // Zh. Org. Khim. 1999.Vol. 35.N 5.
P. 815-816;Rakhimov
A.I., Vostrikova O.V. // The 3d International Ñonference «Chemistry, Technology
and Applications of Fluorocompounds”, CTAF` 2001.June 6-9, 2001. St. Petersburg. Russia.
Abstracts. L4-1. P. 21. 108. Pat. 3133886 US (1964). 109. Ichihara K.,
Aoyama H. // Pat. 1085006 EPO (2001) 110. Skibida I.P.,
Sakharov A.M., Bakhmutov J.L., Denisenkow V.F., Martynova N.P. // Pat. 5495034
US (1996) 111. Kharchuk
V.G., Shishmakov A.B., Volkov V.L., Petrov L.A. // Russ. J. Org. Chem., 1998.
Vol. 34. N 2. P. 284-285; CA1998. Vol. 130. 3595. 112. Zabolotskikh
A.V., Pozdeaeva V.V., Zabolotskikh V.F. . // 1th International Conference
«Chemistry, Technology and Application of Fluorocompounds in Industry», May 30
- June 3, 1994. St. Petersburg. Russia. Abstracts. P4-26. P. 189. 113. Ichihara K.,
Miyamoto M., Baba N., Yoshii S., Homoto Y. // Jpn. Kokai Tokkyo Koho JP
2002053500 (2002); CA2002. Vol. 136. 167083t. 114. Pat. 3478087
US (1969). 115. Pat. 3108131
US (1963). 116. Pat. 2894971 US (1959). 117. Hill M.E.,
Shipp K.G. // Pat. 3526667 US (1970) 118. Furin G.G.,
Il`in A.A., Suchinin V.S., Tolstikova T.G. // Zh.Prikl. Khim., in press. 119. Poster J.J. //
Pat. 347236 US (1968) 120. Nguyen T.,
Wakselman C. // J. Org. Chem. 1989. Vol. 54. N 23. P. 5640. 121. Szlavik Z.,
Tarkanyi G., Skribanek Z., Vass E., Rabai J. // Org. Lett. 2001. Vol. 3. N 15.
P. 2365-2366; CA2001. Vol. 135. 210736b. 122. Furin G.G.,
Il`in A.A., Suchinin V.S., Tolstikova T.G. // Zh. Prikl. Khim., in press. 123. Furin G.G.,
Il`in A.A., Suchinin V.S., Tolstikova T.G. // Zh.Prikl. Khim., in press.
Fluorine Notes, 2004, 33, 1-2
