Fluorine Notes, 2004, 33, 3-4
Joint Fluorination of Alkansulfofluorides and Tertiary Amines Illustrated by Example of Octanesulfofluoride and Ethylbenzenesulfofluoride.
V.A. Matalin, G.I. Kaurova, D.D. Moldavsky, V.G. Barabanov
Russian Scientific Centre "Applied Chemistry",
197198, Russia, Saint-Petersburg, Dobrolubova
avenue, 14
Introduction.
Due to their unique properties perfluorinated organic compounds attract the attention of different lines of industry, including instrument-making and engineering industries, metallurgy, medical and chemical industries. They can be used in many fields of science and technology. However the high cost of perfluorinated products impedes their wide distribution and application.
The working out of more economically sound, low cost and simple obtaining technology of perfluorinated organic compounds, both possessing functional groups or heteroatom in their composition and not possessing them is a very urgent problem. The electrochemical fluorination method is a most preferable one to obtain these compounds, because unlike other perfluorinated compounds obtaining methods it has a number of advantages. The first advantage is an opportunity to obtain perfluorinated organic compounds preserving the original functional group and also to fluorinate (rather successfully) organic compounds with rather long hydrocarbon radicals ( C=7 - 8). The second weighty but not lesser argument in favour of electrochemical fluorination is the fact, that exhaustive or full fluorination passes till isolation of fully fluorinated compound in one apparatus named electrolyzer. The third advantage is that anhydrous hydrogen fluoride is directly used as fluorine source and therefore synthesis phases of elemental fluorine or its carriers, for example cobalt trifluoride, are excluded.
Before the main perfluoroalkanes synthesis method was the hydrocarbon fluorination method using elemental
fluorine or its carriers. However, the late works regarding obtaining of per fluoromethylcyclohexane
and perfluoropentane and others show, that perfluoroalkanes can be obtained using electrochemical
fluorination method and often they are obtained with higher yields.
Most perspective and
popular in demand perfluorinated organic compounds for industrial application are perfluoroalkansulfofluorides
and perfluorinated tertiary amines.
Perfluoroalkansulfofluorides are used as source material for surfactants synthesis, as mixture component in fire-fighting compositions, as source material at synthesis of perspective electrolytes for chemical sources of current [1-3]. Fluorine-containing surfactants are very effective agents, greatly lowering the surface tension at their low content in solution. Surfactants are characterized by high resistance, and perfluorinated derivatives of alkansulfo acids can form stable foam even in the presence of strong oxidizer like chromic and sulphuric acids and they sustain high temperatures [2,3]. Due to these characteristics surfactants are used in fire-fighting compositions, in pools of electrochemical metal plating and at electrochemical refining of nickel and chrome.
Perfluoroalkansulfofluorides are source material for obtaining of perfluoroalkansulfoacids, which
are super acids and are used for organic synthesis. .
Perfluorinated tertiary amines
field application is no less wide. They are known and popular in demand as unique dielectrics-heat
transfers, gas carrying liquids in blood substitutes and in other important fields of science,
technology and economy. One of possible perfluorinated tertiary amines fields of application
is their use as media for transportation of value breed fry.
It is known, that alkansulfofluorides and many other organic compounds do not conduct current in anhydrous hydrogen fluoride. That's why they are subject to electrochemical fluorination in the presence of electrolytic additive.
If sodium fluoride is an electrolytic additive, then that influences the yield of target compound, due to the known influence of NaF on product yield [1], probably because of serious increasing of anodes corrosion and resinification of source organic compounds in electrolyte, that also doesn't make this process commercially paying.
If mercaptans, for example n-bytylmercaptan, are electrolytic additives, then their main drawback
is a strong , obnoxious, specific odor. This fact also prejudices the opportunity of their commercial
use according to the ecological standards. It should also be noted, that mercaptans undergo electrochemical
fluorination very well and their perfluorinated analogues are formed in large quantities in crude
material. As their commercial application is limited, then after isolation of target product
the remaining part of the crude matherial, containing perfluorinated compounds of hexavalent
sulphur, is expulsed that no doubt makes the production process more expensive and less paying.
In our opinion the method that we worked out allows as much simply and effective as possible
to solve this problem and improve a whole number of electrochemical fluorination process indices
among them:
- Electrochemical fluorination process in the presence of tertiary amines (namely triallylamin, triethylamine, tripropylamine, tributylamine and pyridine) as electrolytic additives results in rather high yield of target product and it takes long time. The additive of mentioned amines allows to heighten anodic current density in the electrochemical fluorination process (from 0,001 - 0,002 A/cm2 to 0,03 A/cm2), that greatly increases electrolyzer productivity. The low current densities could have been used to decrease the process of destructive fluorination. However, the application of low current densities decreases the destruction by 5-10% but at that the productivity decreases by 10 ten and more times.
- The application of additives mentioned above allows to carry out the periodical (once in 5-6 hours), but not the continuous organic inflow of electrolyte, which is especially important for industry.
- Widely used in industry, medicine, electrotechnics etc. perfluorinated tertiary amines are formed as by-products of electrolysis.
- Such compounds as perfluorooctane, perfluoropropane, perfluorobutane, which can find or already
have found their application for many industrial branches are formed as by-products (because
of destruction) of reaction..
All above said allows to lower the cost price of perfluorooctansulfofluoride electrochemical synthesis.
Experiments
The experiments were carried out in electrolyzer of Simons type [4] volume equal 0,7 l. Current density was 0,03 A/cm2, initial concentration of octanesulfofluoride (OSF) was 10%, and electrolytic additive was 2,5%. OSF was being added every hour according to calculated expenditure index. The electrolytic additive was added according to the voltage increase, i.e. when it was rising up to 6,5 V, another 2,5% of electricity-conductive compound were added into electrolyte.
At every stage (in 200 A/hours) of electrolysis the analysises of electrolyte for anhydrous hydrogen fluoride and amine content were held, chromatographic analysis of gaseous phase, pouring and crude material analysis were also carried out. The main components of anodic gas were: CF4, NF3, C2F6, C3F8 , SO4F4 etc. An average composition of crude material is listed in table 2.
The main co-reactions passing at anode :
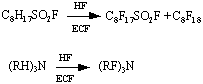
where RH = (C2H5, C3H7, C4H9, C3H5), and also C5H5N - pyridine
Surfactants Synthesis Methods.
Isolated from anhydrous hydrogen fluoride crude material was sent for rectification, where corresponding
fluoroanhydride was isolated with purity equal to 97,0 - 99.0 %. This crude material was rectified
at packed column, filled with glass coils with length and diameter equal to 2 mm, height of column
was 42 cm, diameter 18 mm, the process was carried out at cube temperature in the range st. 70
to 110oC, the residual pressure was changing from 65 to 19 mm Hg. The product was
analyzed using method of GL chromatography. GL chromatography analysis was carried out using
Russian apparatus "Tsvet-100" with thermal conductivity detector, at column, filled with silochrom-80
with 20% ![]() ,
,![]() ,
,![]() -tris-
-tris-![]() -cyanacetophenone.
-cyanacetophenone.
Monomethylethanolamine (MMEA) and monoethylethanolamine (MEEA) was also subject to rectification in vacuum at packed column with the height 42 cm and diameter of 18 mm. The process was controlled using refractive index , which is 1,4390 for monomethylethanolamine and 1,4400 for monoethylethanolamine at 20oC.
Results and discussion
Usually perfluoroctanesulfluoride is obtained by electrochemical fluorination of octanesulfochloride (OSC) or octanesulfofluoride (OSF) :
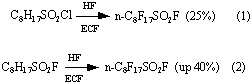
In first case, formula (1), electrolysis with 25% yield takes small period of time, then the
yield greatly decreases, probably because of chlorine participation in the process [5]. This
makes this process commercially unprofitable.
In the second case, which is more close to our process, sodium fluoride or mercaptane (most often
n-butylmercaptan is used) can be used as electrolytic additive, because octanesulfofluoride doesn't
carry current in anhydrous hydrogen fluoride. But this method is characterized by disadvantages,
that were mentioned above.
TABLE 1. MAIN TECHNOLOGICAL CHARACTERISTICS OF OCTANESULFOFLUORIDE AND DIFFERENT TERTIARY AMINES ELECTROCHEMICAL FLUORINATION PROCESSES:
|
The addition of co-fluorinated amine |
|||||
| Electrilysis characteristics | Triallylamine | Triethylamine | Tripropylamine | Tributylamine | Pyridine |
| Addition of OSF, gr. | 327 | 234 | 214 | 83 | 33 |
| Addition of amine, gr. | 115 | 98 | 133 | 132 | 66 |
| Total addition of organics , gr. | 442 | 332 | 347 | 215 | 99 |
| Obtained crude matherial, gr. | 542 | 195 | 285 | 189 | 138 |
| Time of Electrilysis, hours | 106 | 65 | 78 | 69 | 26 |
| Current density, A/cm2 | 0,03 | 0,03 | 0,03 | 0,03 | 0,03 |
| Quantity A*hours | 1584 | 975 | 1133 | 984 | 390 |
| Quantity A*hours/liter | 2400 | 1477 | 1718 | 1490 | 590,5 |
| Yield of crude (current)% | 52 | 37 | 46 | 36 | 58 |
TABLE 2. AVERAGE % CONTENT OF THREE MAIN COB COMPONENTS, WHICH WAS OBTAINED BY
ELECTROLYSIS OF OCTANESULFOFLUORIDE AND DIFFERENT TERTIARY AMINES
|
MAIN CRUDE COMPONENTS, % wt. |
|||
| Amine added | Perfluoroctanesulfyryl fluoride | Perfluoroamine | Perfluorooctane |
| Triethylamine | 42 | 26 | 17 |
| Tripropylamine | 57 | 12 | 20 |
| Tributylamine | 45 | 25 | 12 |
| Triallylamine | 50 | 24 | 14 |
| Pyridine | 15 | 55* | 10 |
*% content of perfluoroamine electrolysis main product is indicated, here perfluoropentane.
As it was mentioned before, ethylbenzene sulfofluoride also was a raw material for perfluoroalkansulfo fluoride synthesis besides octansulfofluoride. We had showed the opportunity of ethylbenzene sulfofluoride and tertiary amines joint fluorination by example of ethylbenzene sulfofluoride and pyridine electrochemical fluorination (table 3).
Table 3. The main technological characteristics of perfluoroethylbenzene sulfofluoride electrochemical synthesis together with pyridine electrochemical fluorination.
| Time of electrolysis (hours) | Current density (A/cm2) | Quantity A*Hours |
Quantity A*hours/l |
Organic mixture addition (g) |
Crude material (g) |
Yield acc. current % |
| 48 | 0,03 | 727 | 1102 | 146 | 234 | 47 |
Besides chromatographic analysis of gas and liquid phase we had carried out the analysis of products obtained by nuclear magnetic resonance method. Obtained data is listed in table 4.
TABLE 4. NMR 19F Spectra of FSO2C8F17 probe (hexafluorobenzene
as internal and external standard)
| N | Shift,
ppm |
Intensity |
Compound structure |
|||
| Probe #1 | Probe #2 | |||||
| Sum | IF | Sum | IF | |||
| 1 | 46,7 | 1,19 | 1,19 | 1,22 | 1,22 | 1 FSO2C12F2C14F2C17F2C18F2C19F2C20F2C21F2C11F3 |
| 2 | 46,6 | 0,26 | 0,26 | 0,11 | 0,11 | (C 7F3)2C24FC13F2C14F2C15F2C16F2C12F2SO22F |
| 3 | -61,2 | 0,11 | 0,27 | |||
| 4 | -67,9 | 0,34 | 0,0 | |||
| 5 | -69,5 | 1,03 | 0,17 | 0,21 | 0,04 | (C 5F3)2C23FC13F2C15F2C16F2 |
| 6 | -70,0 | 0,34 | 0,05 | 0,21 | 0,03 | (C 6F3)2C |
| 7 | -71,4 | 1,44 | 0,24 | 1,09 | 0,18 | (C 7F3)2C24FC13F2C14F2C15F2C16F2C12F2SO22F |
| 8 | -79,7 | 0,34 | 0,11 | 0,16 | 0,05 | C 8F3 |
| 9 | -80,4 | 0,42 | 0,14 | 0,1 | 0,03 | C 9F3 |
| 10 | -80,6 | 0,48 | 0,16 | 0,17 | 0,06 | C 10F3 |
| 11 | -80,7 d 8 Hz | 5,39 | 1,80 | 5,19 | 1,73 | 1 FSO2C12F2C14F2C17F2C18F2C19F2C20F2C21F2C11F3 |
| 12 | -107,4 | 3,82 | 1,91 | 3,93 | 1,96 | |
| 13 | -113,6 | 0,43 | 0,21 | 0,44 | 0,24 | (C 7F3)2C24FC13F2C14F2C15F2C16F2C12F2SO22F |
| 14 | -119,0 | 3,83 | 1,91 | 3,6 | 1,80 | 1 FSO2C12F2C14F2C17F2C18F2C19F2C20F2C21F2C11F3 |
| 15 | -119,2 | 0,52 | 0,26 | 0,42 | 0,21 | (C 7F3)2C24FC13F2C14F2C15F2C16F2C12F2SO22F |
| 16 | -119,8 | 0,50 | 0,25 | 0,4 | 0,2 | |
| 17 | -120,3 | 3,47 | 1,74 | 3,05 | 1,50 | 1 FSO2C12F2C14F2C17F2C18F2C19F2C20F2C21F2C11F3 |
| 18 | -120,5 | 3,47 | 1,74 | 3,05 | 1,50 | |
| 19 | -120,7 | 3,47 | 1,74 | 3,05 | 1,50 | |
| 20 | -121,6 | 3,44 | 1,72 | 3,05 | 1,50 | |
| 21 | -125,4 | 3,70 | 1,85 | 3,39 | 1,70 | |
| 22 | -183,5 d 20 Hz | 0,12 | 0,12 | 0,03 | 0,03 | |
| 23 | -183,9 d 20 Hz | 0,24 | 0,24 | 0,09 | 0,09 | |
| 24 | -185,1 | 0,28 | 0,28 | 0,18 | 0,18 | (C 7F3)2C24FC13F2C14F2C15F2C16F2C12F2SO22F |
1. Two different perfluoroalkylsulfluorides in proportion of 4:1 are discovered in probe, the intensity of signals #1 and #2 indicates this fact. In the probe there are the same alkylsulfofluorides in proportion 9:1.
2. In the probe there is n-perfluorooctanesulfluoride of C11F3C12F2C14F2C17F2C18F2C19F2C20F2C21F2SO21F structure as the main component, to which in spectra signals with the top formula indices correspond.
3. In the probe there are 2-trifluoromethylsubstituted perfluoroalkylsulfluorides, what the signals ## 4-7 indicate, meeting (CF3)2C-group and what the signals ##22-24 indicate, meeting >CF-group at intensity proportion of these signals close to 6:1.
4. In the probe there is iso-perfluorooctanesulfluoride isomer (perfluoro-6-methylheptanesulfluoride with structure (C7F3)2C24FC13F2C14F2C15F2C16F2C12F2SO22F) as second by intensity of signal.
5. Because of signals ##12 and 14 of fluorine atoms chemical shifts of perfluorooctanesulfluoride are close with iso-perfluorooctanesulfluoride signals there intensity are too high relatively signals 17-19.
6. The presence of signals 8-10 typical for CF3-group in spectra and signals 22,23
typical for >CF-group are evidence of isomer presence not only in 6 position but also with
structure
![]()
7. Signal 6 of (CF3)2C-group also can to correspond to isomer structure
with two ramification
![]()
References
1. The role of adsorption in electrochemical perfluorination of alkylsulfofluorides. G. Cauquis, B. Keita and G. Pierre. J. Electroanal. Chem., 100 (1979) 205-215.
2. Organicheskaya ehlektrokhimiya. t. 2. s. 781-788. M. Khimiya. I988;
3. Abe T. Nagase S. Chapter I. Electrochemicalfluorination (Simons process) as a route to perfluorinated organic compounds of industrial interest. 19-43, (I982).
4. Ftor i ego soedineniya. Pod redaktsiej Dzh. Sajmonsa. Izdatel'stvo inostrannoj literatury. Moskva, 1953.
5. N. Ignat`ev, A. Kucherina and P. Sartori. Acta Chemica Scandinavica 53 (1999) 1110 - 1116.
Fluorine Notes, 2004, 33, 3-4
