Fluorine Notes, 2004, 32, 1-2
THE SYNTHESIS AND SOME CHARACTERISTICS OF PARTLY FLUORINATED ALCOHOLS ON THE BASIS OF TETRAFLUOROETHYLENE
AND HEXAFLUOROPROPYLENE
(REVIEW)
G.G. Furin
The Organic Chemistry Institute named after N.N. Vorojtzov of the Novosibirsk city Sibirian department of Russian Academy of Sciences
9, Academic Ak. Lavrentiev avenue, Novosibirsk, 6300090 Russia
E-mail :
furin@nioch.nsc.ru
In this review the approaches of partly fluorinated alcohols on the tetrafluoroethylene and hexafluoropropylene basis are analyzed. New experimental data regarding telomeric alcohols involvement into reactions with unsaturated compounds and hetero-organic derivatives are discussed. Oxidization processes and reactions in strong acid mediums of telomeric alcohols are analyzed. Also in this review you will find the trends of practical using of telomeric alcohols. The questions regarding partly fluorinated alcohols toxicity and some of their derivatives are discussed here.
Table of contents
Introduction. The role of fluorine containing compounds in new fluorine materials creation.
1. The development of partly fluorinated alcohols obtaining technology using interaction of teterafluoroethylene and hexafluoropropylene with alcohols in the presence of radical initiators.
2. Partly fluorinated alcohols as effective O-nucleophilic reagents, their application for creating of fluorine containing semi-products and materials on their basis.
2.1. The interaction of trimethylsilyl ethers of partly fluorinated alcohols with unsaturated compounds – the synthesis way of wide application field dialkyl ethers.
2.2. The reactions of polyfluoroaromatic compounds with telomeric alcohols and creating of new materials on their basis.
2.3. The interaction of telomeric alcohols with hetero-organic compounds.
2.4. The oxidization of telomeric alcohols till polyfluorinated carboxylic acids and their (acids) practical use.
2.5. Dielectric heat-transfers synthesis on the base of partly fluorinated alcohols.
2.6. The processes with telomeric alcohols participation.
3. The toxicity of partly fluorinated alcohols and some derivatives.
References
2. Partly Fluorinated Alcohols as Effective O-nucleophilic reagents, their application for creation of fluorine containing semi-products and materials on their base.
On the basis of alcohols interaction with tetrafluoroethylene and hexafluoropropylene in the liquid phase in the presence of radical sources universal complex obtaining technology of semi-products (poly-fluorinated alcohols, the most important components of fluoroorganic materials production) was developed and is functioning successfully. They can be parent materials for production of many fluorocontaining materials, used in engineering industry and medicine. In connection with this the functionalization of telomeric alcohols like H(CF2CF2)nCH2OH, n = 1-5 widens the production fields of new perspective fluorine materials. Below you will find some directions of its realization.
Thus on their basis new materials with important chemical and physical characteristics are created. These researches opened excellent features of a number of new emulsions regarding their ability to storage, sterilization and rheology. As time goes by they will become a basis of alternative replacement of industrial liquids and they will find a wide range of application.
Poly-fluorinated alcohols are more acidic compare to hydrocarbons analogues,
that results in reduction of their nucleophilic properties (table 2) [56].
Table 2.Constants of some fluoroalchols acidity at 25 oC [56].
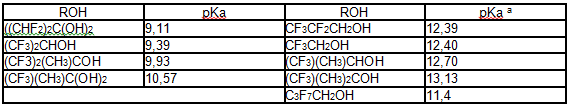
a [57].
The peculiarity of telomeric alcohols chemical behavior is their higher acidity and reduced reaction ability as nucleophilic reagents. Nevertheless, they were used in nucleophilic replacement and addition reactions. For example , 2,2,3,3-tetrafluoropropanol reacts in autoclave with tetrafluoroethylene in the presence of NaOH in dioxane at 60oC during two hours, producing CHF2CF2CH2OCF=CF2 with the yield of 42 % [58].
2.1. The interaction of trimethylsilyl ethers of partly fluorinated alcohols and unsaturated compounds - the way of wide application field dialkyl ethers synthesis.
One of the approaches of partly fluorinated alcohols activation as O-nucleophiles was their trimethylsilyl ethers use in the process carrying out conditions in the presence of fluoride-ion source. Cesium fluoride is rather effective source. Thus, this method is used for forming of C-O and C-N bonds in highly fluorinated systems [48,49]. This method is successfully used instead of olefins reactions with alcoholates of alkali elements.
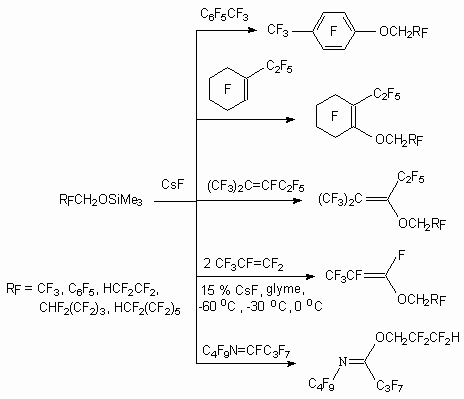
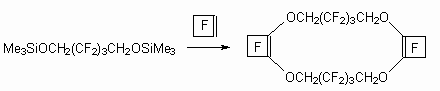
The alcohols' reaction H(CF2CF2)nCH2OH (n = 1-3) with hexafluorobenzene and octafluorotoluene in the presence of CsF, Et3N or pyridine results in formation of replacement products of fluorine atoms in benzene ring [61]. In case of trimethylsilyl ethers of telomeric alcohols reaction with mixture of isomeric trimers of hexafluoropropylene two butadiene derivatives are formed.
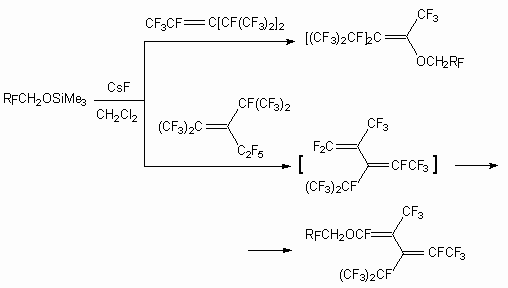
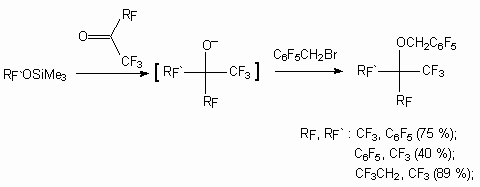
For reactions with pentafluoropyridine, pentafluorosubstituted benzenes like C6F5X (X = CN, CF3, Cl, F) , 1,4-diiodotetrafluorobenzene in the conditions of catalysis using fluoride-ion the following trimethylsilyl ethers of fluorocontaining alcohols are used: (CF3)2CHOSiMe3, (CF3)3COSiMe3, CH3C(CF3)2OSiMe3, PhC(CF3)2OSiMe3, C7F17CH2CH2SiMe3, CF3CH2OSiMe3, CF3C(CH3)2OSiMe3, (CF2CH2OSiMe3)2, CF2(CF2CH2OSiMe3)2 [62]. Likewise such ethers are introduced into reaction with tribromomethane.
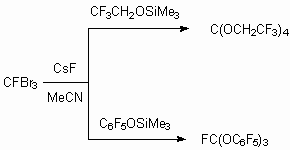
Reactions with fluorocontaining ketones were put into practice, resulting in formation of perfluorinated alcohols, which are transferred into corresponding perfluorinated ethers [62].
The reactions of bifunctional trimethylsilyl ethers of telomeric alcohols with polyfluorinated benzenes and pentafluorobenzylbromide in diglyme produce replacement products of fluorine atoms in benzene ring and bromine of CH2Br group [63].
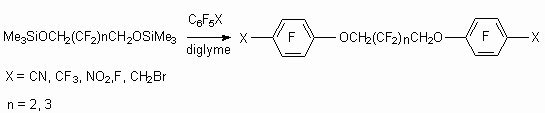
These compounds are liquids with freezing points -20 oC and boiling points 330-350 oC and they can be used as dielectric heat transfers with wide application field.
Compounds, possessing movable halogen atoms, also react in such reaction with formation of halogen atoms replacement products, besides this the formation of heterocyclic compounds exists [64].
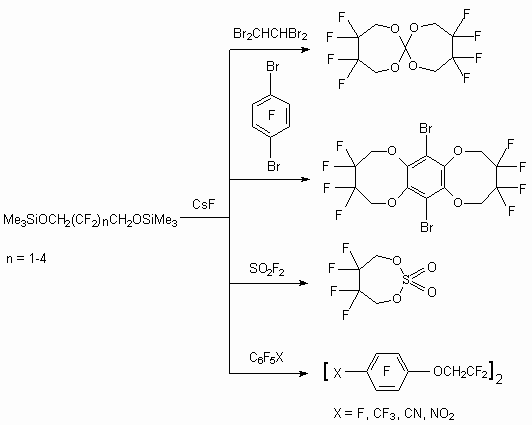
In the case of perfluorocyclic olefins the replacement of both fluorine atoms takes place at multiply bond with formation of polycyclic compound. It should be noted, that cycles like these can act as complex formers like crown ethers, for example with fluorine anion [65].
This method doesn't have any limits for practical purposes. Thus compounds, having movable fluorine atoms at phosphorus atom, are introduced into the reaction [66].
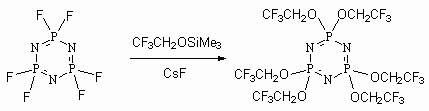
Polyfluoroalkoxytrimethylsilanes react with compounds of quinquivalent phosphorus with formation of polyfluoroalkyliodids [67]. This is an interesting variant of transfer from telomeric alcohols to polyfluoroalkyliodids. The key moment of this reaction is the formation of stable triphenylphosphine oxide.
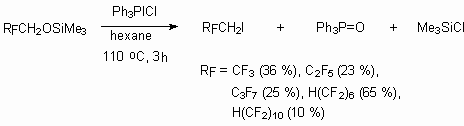
The reactions of telomeric alcohols with polyfluoroaromatic compounds in the presence of KOH results in formation of di- and tri-substituted products, which can be used as high temperature (more than 350 oC) liquids, used as lubricants, heat transfers and hydraulic liquids [68]. The demand of high temperature lubricants, which at moving details processing increase the mechanisms' function life due to their low constant of friction, is rather high. Along with this they can be used as lubricants for rolling mills of both steel and other metals. Another field of application is for oil equipment and oil lines, because such lubricants are not washed off with organic compounds compare to mineral oils.
Partly fluorinated alcohols reacts with other benzene polyfluoroderivatives: with 2,3,4,5,6-pentafluorostyrol /NaH [69], decafluoroazobenzene/CsF [70], deca-fluor-m-dimethylbenzene/NaH [71] with formation of replacement products of benzene ring fluorine atoms.
2.3. The interaction of telomeric alcohols and hetero-organic compounds.
Techonologically available 2,2,3,3-tetrafluoropropyl alcohol is used for synthesis of volatile xanthogenates of metals [72]. Thus the reaction of this telomeric alcohol with carbon bisulfide in absolute diethyl ether at 0 oC in the presence of KOH results in formation of potassium salt of 2,2,3,3-.tetrafluoropropylxanthogenate, which later under the influence of nickel, cobalt, palladium salts produces corresponding salts.
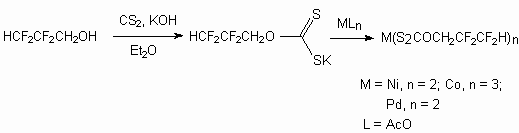
Telomeric alcohols can be used in the following perspective directions. First of all, they can be used for flotation of gold and silver containing minerals. [73]. Thus at flotation of clay- silica ores the use of such telomeric alcohols allows to increase the gold yield at the rate of 8.9% and silver yield at the rate of 21.7% compare to use of potassium xanthogenate
Potassium 1,1,2,2-tetrafluoroethylxanthogenate was used at flotation of sulphide ores as collector for flotation separation of polymetallic concentrates [74].
Polyfluoroorganosilicon monomers form oil and water repellent bio-resistant coatings
on the surface of different materials. Their production is based on the interaction
of  ,
, ,
, -trihydroperfluoroalkane
alcohols like H(CF2CF2)nCH2OH or polyfluorohalogenalkanes
like H(CF2CF2)nCH2X (X = Cl, Br) with
silicium compounds [75].
-trihydroperfluoroalkane
alcohols like H(CF2CF2)nCH2OH or polyfluorohalogenalkanes
like H(CF2CF2)nCH2X (X = Cl, Br) with
silicium compounds [75].
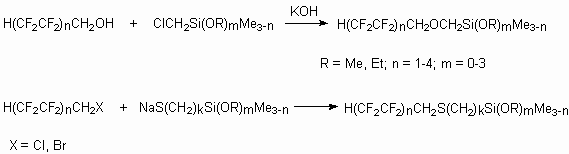
From the last compounds were obtained corresponding siloxanes having useful technical characteristics .
Deeply fluorinated di-epoxides are obtained at the reacion of alcohols like HCH2(CF2)nCH2OH (n = 3, 8) with epichlorohydrin in the basic media in the presence of tetrabutylammonium hydrosulphate as catalyst [76].
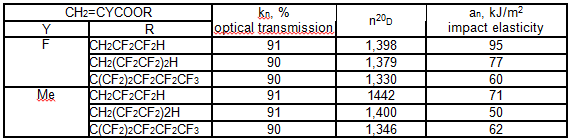
It turned out, that fragment H(CF2CF2)nCH2O in the polymer composition can call changes of its properties. Thus, modified polymethylmethacrylate especially co-polymer 1,1,3-trihydrotetrafluoropropyl-2-fluoroacrylate with 1,1,5-trihydrooctafluoroamylacrylate possess the properties of light guide [77].
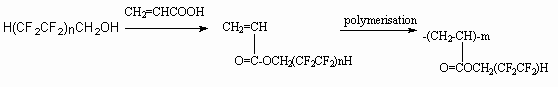
The polymer light guide index of refraction is rather low, the melt flow index for light reflecting cover applying is 10-30 g/10 min at 190 oC. They can be used for light guides production.
The obtaining of acrylic acid polyfluorinated esters can be carried out either by interaction of acrylic acid with telomeric alcohol in the presence of acid (for example, meta-toluenesulpho- acid), or by using of Prince reaction [78-80].

Fluorine anhydride of  -fluoroacryl
acid, which is transferred into esters by reaction with telomeric alcohols,
is obtained using interaction of 2,2,3,3-tetrafluorooxetane, halides of alkali
metals and dehalogenating agent in aprotic bipolar solvents in the presence
of radical polymerization inhibitor [78-80].
-fluoroacryl
acid, which is transferred into esters by reaction with telomeric alcohols,
is obtained using interaction of 2,2,3,3-tetrafluorooxetane, halides of alkali
metals and dehalogenating agent in aprotic bipolar solvents in the presence
of radical polymerization inhibitor [78-80].

Polymers of  -fluoroacrylates
noticeably exceed their meta-acrylic analogues in many operating parameters.
Fluorine introduction into polymer ester part resulted in improvement of
block organic glass properties and first of all in heat resistance and impact
resistance. Excellent optical properties of such fluorine modified polymers
in combination with high heat resistance, flexibility and water-resistance
allow to use them in fiber optics. Refraction index of such polymers equal
to 1.36 - 1.50 allows to use them as materials of light guide body and reflective
cover [81-89]. They can be used as materials for optical storage devices
of optical disks [84,90].
-fluoroacrylates
noticeably exceed their meta-acrylic analogues in many operating parameters.
Fluorine introduction into polymer ester part resulted in improvement of
block organic glass properties and first of all in heat resistance and impact
resistance. Excellent optical properties of such fluorine modified polymers
in combination with high heat resistance, flexibility and water-resistance
allow to use them in fiber optics. Refraction index of such polymers equal
to 1.36 - 1.50 allows to use them as materials of light guide body and reflective
cover [81-89]. They can be used as materials for optical storage devices
of optical disks [84,90].
Bis(fluoroalkyl)alkylphosphonats are obtained with the yield of 28-69 % at
treatment of Cl2P(O)R using telomeric alcohols in the presence
of triethylamine [91]. Such compounds are used as hydraulic liquids.
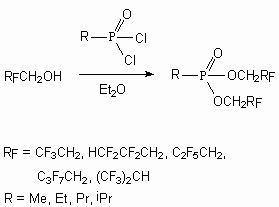
The reaction of phosphorus trichloride with alcohols like RFOH ( RF = HCF2CH2, H(CF2)2CH2, H(CF2)4CH2, CF3CH2, C2F5CH2, C3F7CH2, (CF3)2CH, C6F13CH2CH2, CF3Me2C, (CF3)2MeC, CF3CH2CH2 ) results in formation of phosphorus derivatives like (RFO)2P(O)H with the yield equal to 42-89 % [92]. These compounds are more thermally stable compare to hydrocarbon analogues.
The important moment of telomeric alcohols interaction with phosphorus compounds was the synthesis of polyfluorohalogenalkanes - fluoroorganic semi-products [75]. Thus the polyfluorohalogenalkanes obtaining method by action of PCl3 , PCl5 (or PBr5) onto telomeric alcohols was developed.
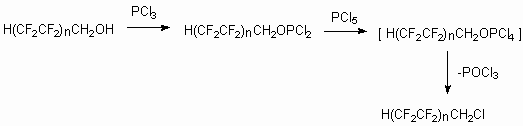
The transformation of telomeric alcohols H(CF2CF2)n CH2OH
(n = 1-5) into other semi-products, out of which important materials for
techniques and medicine can be obtained, is an important way of fluorine
containing materials synthesis.
The use of telomeric alcohols' H(CF2CF2)n
CH2OH (n = 1-5) oxidization processes under the influence of halogens
for creation of the new generation of blood substitutes and perfluorinated
dicarboxylic acids.
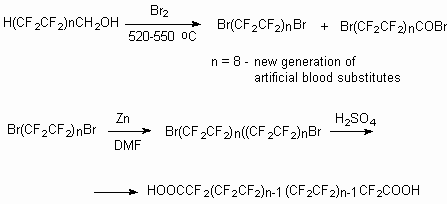
Later these semi-products are subject to transformation into hard-to-reach compounds, which now are in stable demand.
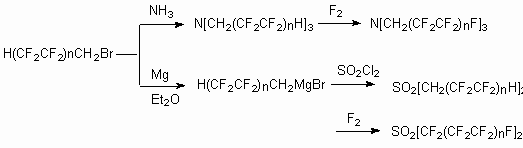
These compounds can be used as high temperature dielectrics, heat transfers, high parameters hydraulic liquids for electric machines, airplanes and aircrafts hydraulic systems. As these compounds are incompressible they can find their application as hydraulic liquids for robotics and they can be effective lubricating oils for machines' and mechanisms assemblies.
In few cases the introduction of H(CF2CF2)nCH2O
group into molecule essentially influences the stability of organic molecule.
Thus, the authors of works [93,94] for the first time have put into practice
the synthesis of stable organic compounds of quatrovalent quatrocoordinated
sulphur. Such model compounds with  ,
,
 ,
, -trihydropolyfluoroalkoxyl
group turned out to be rather stable compounds.
-trihydropolyfluoroalkoxyl
group turned out to be rather stable compounds.
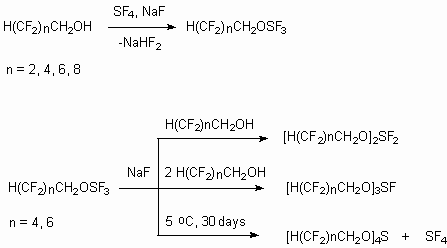
Sulforanes possess polyfluoroalkylying properties, react with nucleophilic reagents, act as fluorinating and dehydrating reagents [95-98].
It is stated [99,100], that carrying out of 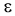 -caprolactam
reaction with 1,1,3-trihydroperfluoropropanol in DMF and trimethylamin results
in formation of esters of
-caprolactam
reaction with 1,1,3-trihydroperfluoropropanol in DMF and trimethylamin results
in formation of esters of  -aminocaproic
acid oligomers with the yield equal to 36% (during 4 hours at 220-260 oC
). Poly-fluorinated esters of
-aminocaproic
acid oligomers with the yield equal to 36% (during 4 hours at 220-260 oC
). Poly-fluorinated esters of  -aminocaproic
acid oligomers can be used as metals' protection material against corrosion,
as anticorrosion hydrophobic coats for protection of articles surface against
aggressive medias, they also might be perspective regards antiviral activity.
They are easily put on different surfaces in the form of varnishes.
-aminocaproic
acid oligomers can be used as metals' protection material against corrosion,
as anticorrosion hydrophobic coats for protection of articles surface against
aggressive medias, they also might be perspective regards antiviral activity.
They are easily put on different surfaces in the form of varnishes.
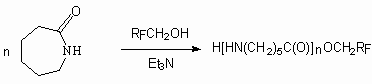
As the length of telomeric alcohol carbon chain increases the yield of dehydrofluorination and polymerization by-products increases also. Thus for H(CF2CF2)nCH2OH prolonged inductive period (more than 60 minutes) is observed, the oligomer yield for the period of 4 hours is 61% at fractional conversion equal to 74%. In this process the solvent is used as catalyst. Copper acetate appeared to be more effective as catalyst.
The authors of work [101] showed the opportunity of associative interactions of polycaproamide with telomeric alcohols H(CF2CF2)nCH2OH (n = 1,2). Polycaproamide forms homogeneous solutions at 15 oC (n = 1, during 18 min, n = 2 , during 37 min) with concentration of polymer equal to 0.1-3 %. At heating up to 50-80 oC the concentration rises up to 15 %, and at boiling point of alcohol - up to 25 %. Such polycaproamide modified by telomeric alcohols reveals higher thermal stability and can be recommended for use in compositions with lowered flammability, in mechanical rubber articles with higher aggressive resistance and resistance to gasoline and also for obtaining articles with higher wearing qualities.
Ketones and aldehydes are subject to oxidization according to Baeyer-Villiger
type using hydrogen peroxide in the presence of catalyst in the media of
fluorinated alcohols R1R2R3COH (R1-R3 = CnF2n+1, H, n = 1-6) . Thus, the mixture of cyclohexanone,
1,1,1,3,3,3-hexafluoropropan-2-ol and para-toluenesulfonic acid
under action of hydrogen peroxide at 60 oC during 65 min produce
 -caprolactam
with 99 % yield [102].
-caprolactam
with 99 % yield [102].
Telomeric alcohols are used for obtaining of different derivatives due to the fact that the transformation of alcohol group take places. Thus, using sulfoesters they were turned into trialkylamines with high stereoselectivity and high effectiveness [103].
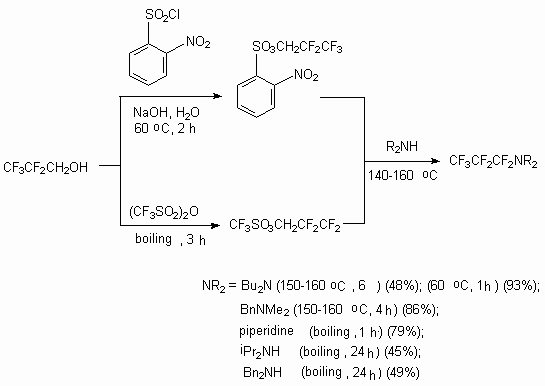
Such amines at action of bases transform into  -(trifluoromethyl)-
-(trifluoromethyl)- ,
, -unsaturated
amines.
-unsaturated
amines.
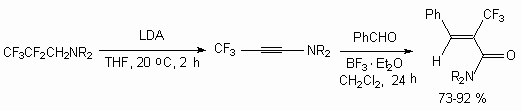
Polyfluoroalkylchlorosylphites synthesis out of available for industry telomeric alcohols by their treatment with sulfuryl chloride and thionyl chloride was worked out [23]. The reaction doesn't run when without catalysts, but goes in the presence of tertiary amines (triethylamine, urotropine and others). It is stated, that initially complexes of structure H(CF2CF2)nCH2O-*NHEt3 are formed (heat of formation for n = 1 complex is 19.8 kJ/mol, for n = 2 - 21.6 kJ/mol). At treatment of last mentioned with thionyl chloride the yield of polyfluoroalkylchlorosulphites H(CF2CF2)nCH2S(O)Cl reaches more than 80 % at conversion of alcohol more than 92 % [23]. The constants of reaction speeds are determined, that reactions run according to the second order. The reaction is carried out in the media of inert solvent (CHCl3, CCl4). To depress the formation of bis-polyfluoroalkylsulphite it is necessary to use the 1.5-2.5 mol of thionyl chloride excess.
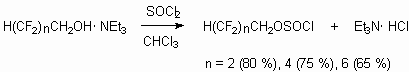
Polyfluoroalkylcholosulphites in contrast to non-fluorinated analogues are resistant compounds up to 100 oC. The rate constant of decomposition reaction is determined as first order. The authors explain this as particularities of internal molecule associative interactions of CH2 group hydrogen atom with chlorosulphite group oxygen, which makes difficult the attack of electrophilic carbonic atom of chlorine, that usually results in destruction with extraction of sulfurous anhydride and formation of chloroalkane. Telomeric alcohols' reaction with sulfuryl chloride the corresponding polyfluoroalkylfluorosuphates, which are new polyfluoroorganic syntones are obtained [75].
Fluorosulphates are colorless easy movable resistant to liquid hydrolysis (n
<8) or solid compounds (n > 8) stable at prolonged storage. Their most
important characteristic is turning into fluoroanhydrides of  -hydroperfluorocarboxylic
acids at treatment using metal fluorides. This is used as obtaining method
of poly-fluorinated carboxylic acids [104].
-hydroperfluorocarboxylic
acids at treatment using metal fluorides. This is used as obtaining method
of poly-fluorinated carboxylic acids [104].
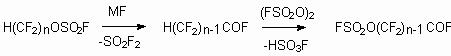
The further hydrogen replacement for FSO2O group results in formation
of  -(fluorosulfonyloxy)perfluoroacylfluorides,
the initial semi-products for production of ion-exchange membranes [105].
-(fluorosulfonyloxy)perfluoroacylfluorides,
the initial semi-products for production of ion-exchange membranes [105].
Polyfluoroalkylchlorosulphites and polyfluoroalkylchlorosulphates are used as alkylating reagents for monomers' production, as hydraulic liquids, heat transfers and surface active materials. It is stated that, reaction of fluorinated telomeric alcohols with alcohols is catalyzed by metal oxides of 2-nd and 3-d groups and proceeds through formation of glycols and polyfluoroalcoholates of metals. The reaction products are used for monomres' production and peroxides initiators. [106].
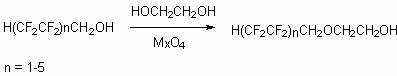
The reaction rate increases twice at change from telomeric alcohols n=1,2 to telomeric alcohols n=3-5.
Polyfluoroalkylchlorosulphites easily react with KF, LiCl, KBr, KI with formation
of corresponding polyfluoroalkylhalogenides with the yield of 75-90 %. The
interaction of polyfluoroalkylchlorosulphites with the salts of acrylic and
methacrylic acids results in obtaining of corresponding esters with the yield
of 80 % [107]. On the basis of these monomers the co-polymer for contact
eye-lenses is obtained.
to be continued
Fluorine Notes, 2004, 32, 1-2
