Fluorine Notes, 2003, 31, 1-2
THE SYNTHESIS AND SOME CHARACTERISTICS OF PARTLY FLUORINATED ALCOHOLS ON THE BASIS OF TETRAFLUOROETHYLENE
AND HEXAFLUOROPROPYLENE
(REVIEW)
G.G. Furin
The Organic Chemistry Institute named after N.N. Vorojtzov of the Novosibirsk city Sibirian department of Russian Academy of Sciences
9, Academic Ak. Lavrentiev avenue, Novosibirsk, 6300090 Russia
E-mail : furin@nioch.nsc.ru
In this review the approaches of partly fluorinated alcohols on the tetrafluoroethylene and hexafluoropropylene basis are analyzed. New experimental data regarding telomeric alcohols involvement into reactions with unsaturated compounds and hetero-organic derivatives are discussed. Oxidization processes and reactions in strong acid mediums of telomeric alcohols are analyzed. Also in this review you will find the trends of practical using of telomeric alcohols. The questions regarding partly fluorinated alcohols toxicity and some of their derivatives are discussed here.
Table of contents
Introduction. The role of fluorine containing compounds in new fluorine materials creation.
1. The development of partly fluorinated alcohols obtaining technology using interaction of teterafluoroethylene and hexafluoropropylene with alcohols in the presence of radical initiators.
2. Partly fluorinated alcohols as effective O-nucleophilic reagents, their application for creating of fluorine containing semi-products and materials on their basis.
2.1. The interaction of trimethylsilyl ethers of partly fluorinated alcohols with unsaturated compounds – the synthesis way of wide application field dialkyl ethers.
2.2. The reactions of polyfluoroaromatic compounds with telomeric alcohols and creating of new materials on their basis.
2.3. The interaction of telomeric alcohols with hetero-organic compounds.
2.4. The oxidization of telomeric alcohols till polyfluorinated carboxylic acids and their (acids) practical use.
2.5. Dielectric heat-transfers synthesis on the base of partly fluorinated alcohols.
2.6. The processes with telomeric alcohols participation.
3. The toxicity of partly fluorinated alcohols and some derivatives.
References
Introduction. The role of fluorine containing compounds in creation of new fluorine materials.
The data of recent theoretical and experimental researches allowed to develop the syntheses' methods and production technologies of previously unavailable or unknown compounds and also to intensify a lot of known reactions, especially obtaining methods of ozone friendly chladones (freons). This data made the synthesis of previously exotic compounds technologically simple, effective, high selective and safe.
It is stated, that perfluorinated organic compounds can be successfully used as inert solvents and as medias for impact- sensitive explosive material's components. It is also stated, that they can find their application for the processes, proceeding with participation of high reaction reagents and reagents possessing oxidizing properties. A number of semi-products is used at creation of high temperature and hydrophobic additives for lubricating oils, antiadhesive and antiwear additives, and also as syntones for building of complicated fluorine containing molecules.
The production of several ozone friendly chladones (R 125, 218, 227, 318 and etc) is arranged on the basis of teterafluoroethylene and hexafluoropropylene. The obtaining, purification and extracting technologies of a number of perfluorinated organic compounds are developed, it allows to create a base for supplying industry and medicine with high quality and available perfluorinated materials: perspective dielectrics-heat transfers, actuating fluids for power engineering, astronomic, aviation and navigation techniques, blood substitutes components, liquids for conservation of body organs, new ozone-safe freons, satisfying the increased consumer properties and ecology.
Perfluorinated organic compounds due to their thermal and electrophysical characteristics find their application as new dielectrics in electric facilities and machines. They can successfully replace sulfur hexafluoride, essentially decreasing the frame of electric facilities and simplifying the facilities. Here not only high characteristics of electric strength and low electroconductivity are important, but also high thermal characteristics, typical for the liquid state of the compound. The requirements, applied to radio electronic equipment and electric machines, which nomenclature is extremely wide, are rather rigid and have a sharp tendency for further toughening. First of all, it is microminiaturization, and thus, the increasing of electric field local strength, heat flows and power of high frequency field. The solving of these tasks is closely connected with creation of dielectric heat-transfers on the basis of perfluorinated organic compounds, used both in liquid and vapour phase. This requires rapt attention to class of perfluorinated organic compounds and detailed study of their physical, and especially electrophysical properties. It should be stressed, that in many cases these characteristics are specific for fluorine compounds and do not reveal themselves in compounds of other elements.
The advantages of perfluorinated organic compounds, except high electric strength are low viscosity, incombustibility, non-toxicity, high pressure of vapour, that makes them convenient in working. Their use in mode of evaporating cooling lowers in 10 and more times the mass-volume characteristics of radio electronic equipment, lowers power inputs to provide the cooling of 100-1000 times, increases the durability of facility functioning. The application of liquid dielectrics on their basis is rather perspective for transformers and electric machines, used in coil-mining and chemical industries, such application provides durability of equipment and increases fire and explosion safety of their operation. Besides this, the low freezing points allow to improve operating characteristics of equipment for Far North regions with long influence of low temperatures.
Although the scale of such compounds application as dielectrics and heat-transfers in dc voltage
facilities is not yet great. It is not quite clear for now, if electric strength is a liquid
internal characteristic of media or a consequence of pre-breakdown processes and can change
with considerable range at change of process nature. Only the permittivity - the one characteristic
out of three main liquid dielectric characteristics (permittivity 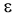 ,
electroconductivity
,
electroconductivity 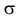 and electric strength E) is a true characteristic of liquids. Electroconductivity is determined
by ionogenic admixture availability, liquid preparation method and electrode reactions. Electric
strength is also not so much a physical as a technological characteristic of liquid and electrodes.
That's why it is rather important for liquid preparation technology and technological factors
to influence the electric strength of liquids.
and electric strength E) is a true characteristic of liquids. Electroconductivity is determined
by ionogenic admixture availability, liquid preparation method and electrode reactions. Electric
strength is also not so much a physical as a technological characteristic of liquid and electrodes.
That's why it is rather important for liquid preparation technology and technological factors
to influence the electric strength of liquids.
Perfluorinated ethers are a class of compounds with interesting properties. Thus the entire replacement of hydrogen atoms for fluorine in carbon chain results in increasing of thermal resistance and stability to oxidizers action, freezing point lowering, improving of molecules' thermal and electrophysical characteristics in a whole, increasing of lubricating properties [1-3].
These properties allow using of perfluorinated dialkyl ethers as liquid dielectrics, heat transfers,
lubricants, inert liquids, the so- called oxygen transfers. They are good solvents, they
are used in electronic industry and also for body organs preservation.
The main methods
of this class compounds synthesis are based on fluorination processes. This is a fluorination
of hydrocarbon analogues. Although the target products' yield using this method is rather
low. For example, the fluorination using elemental fluorine of monoglyme produces perfluorinated
products with yield of 21% and 16% respectively [4]. The yield is just as low at electrochemical
fluorination. At the same time using the apparatus of a special structure perfluorinated
dialkyl ethers having branched radicals with good yield was obtained [5]. The majority of
perfluorinated crown ethers are obtained using this method [5].
The other method is the fluorination of polyethers, when along with the fluorination process the destructive separation of polymer chain takes palace [1].
Recently the most successful methodology of perfluorinated dialkyl ethers' synthesis was the fluorination of partly fluorinated ethers, obtained by alcohols' action on perfluoroolefines [6]. The increasing of thermal stability at introduction of fluorine atoms of such basic materials results in essential increasing of target entirely fluorinated products yield at the expense of lowering of destructive processes and controlled depth of fluorination. We think, that this methodology has essential advantages and allows to obtain different perfluorinated dialkyl ethers with high economy.
Partly fluorinated alcohols started to play an important role in the development of fluoroorganic materials chemistry. The present review is based on discussion of their synthesis and properties.
Partly fluorinated primary alcohols like H(CF2CF2)nCH2OH (n = 1-6), the production of which is based on interaction of tetrafluoroethylene with methyl alcohol at increased temperature and pressure in the presence of peroxide initiator with decomposition point higher than 100 oC (tert-butyl diperoxide,tert-butylperoxy-2-ethylhexanoate , carboxylic acids peroxides, ethers peroxides, 2,5-bis-(tert-butylperoxy)-2,5-dimethylhexane and others) [7-21]. Thus alcohols ROH (R = CHCF2CF2CH2, CF3CHFCF2CH2) are obtained by heating of peroxide initiator (3,5-15,4 %) in alcohol at 110-120 oC and pressure of 3,4-6,5 bar with control of fluoroolefine introduction and increasing of pressure up to 9 - 15 bar at 150 oC . The process is carried out at proportion of fluoroolefine / initiator (7.2 - 28.1 ) / 1 and in presence of 0.2-1.2 % polyfluorinated alcohol [8]. At these conditions the initiator decompose to radical and its reaction with alcohol is induced. For example, CHF2CF2CH2OH with the yield of 99.8 % is obtained at reaction of methyl alcohol and tetrafluoroethylene in the presence of di(tert-butyl)peroxide [8].
The radical telomerization of tetrafluoroethylene with methyl alcohol regardless of the initiator's
nature results in formation of mixture consisting of 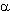 ,
, 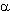 ,
,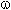 -trihydroperfluoroalcohols
and their isomers H(CF2)nCH(OH)(CF2)mH,
where n and m are numbers, divisible by 2. There are no indications regarding dependence
of formed products' structure on used initiator. In the work [22] it was stated,
that telomerization of tetrafluoroethylene using methyl alcohol at temperature within
65-75 oC and pressure equal to 15-18 bar using hydrogen peroxide as initiator
results in formation of mixture consisting of 1,1,4-trihydroperfluorobutanol and
1,1,6-trihydroperfluorohexanol (alcohols with odd number of difluoromethylene groups)
along with telomeric alcohols. Although the yield of these products is extremely
low and amounts to 0.5-2 % of overall alcohols' sum. The formation of these products
the authors account for the proceeding of process according to the following scheme:
-trihydroperfluoroalcohols
and their isomers H(CF2)nCH(OH)(CF2)mH,
where n and m are numbers, divisible by 2. There are no indications regarding dependence
of formed products' structure on used initiator. In the work [22] it was stated,
that telomerization of tetrafluoroethylene using methyl alcohol at temperature within
65-75 oC and pressure equal to 15-18 bar using hydrogen peroxide as initiator
results in formation of mixture consisting of 1,1,4-trihydroperfluorobutanol and
1,1,6-trihydroperfluorohexanol (alcohols with odd number of difluoromethylene groups)
along with telomeric alcohols. Although the yield of these products is extremely
low and amounts to 0.5-2 % of overall alcohols' sum. The formation of these products
the authors account for the proceeding of process according to the following scheme:
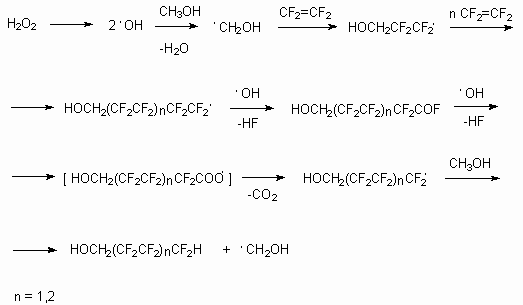
Telomeric alcohols have found a wide application in fluoroorganic synthesis [23]. Thus, they are used for production of many fluorine containing materials and they also are semi-products first of all for production partly fluorinated carboxylic [24-28] and -alkansulfine [23] acids, fluorinated ethers of glycols (via CH2OH and CHF2- groups)[23]. At the same time they in the presence of base act as active O-nucleophiles, which allow to receive the entire spectrum of ethers and esters [1]. The interest to the development of definitely specified alcohol obtaining technologies has sharply raised in recent years, when the effectiveness of their use at production of information-reproducing medias with reproducing layer able to read laser information was shown, and also it was shown in production of high effective surface active materials and photo developing compounds in photography. [29].
It is stated [30], that in addition to methyl alcohol other alcohols can be introduced into reaction of tetrafluoroethylene and hexafluoroethylene. At that the conditions of the process carrying out do not change (temperature 125oC, pressure 0.8 MPa, radical initiator di-tert-butylperoxide). Although the conversion is not high (20.2 %) high selectivity is observed (95 % and more). In new patents the obtaining method of fluoroalkanols is based on the main process - the radical addition of methyl alcohol to tetrafluoroethylene [8]. The difference of patents is brought to optimization of process of 2,2,3,3-tetrafluoropropanol producing. It turned out [31-40], that process carrying out is more effective in the presence of CaCO3. If at that the process temperature is kept within 60-65 oC (4 hours), then 2,2,3,3-tetrafluoropropanol [32] is formed, while at 125 oC and pressure equal to 0.8 bar (6 hours) the mixture of telomeric alcohols is obtained [32]. The best results for synthesis of 2,2,3,3-tetrafluropropyl alcohol were obtained at proportion: tetrafluoroethylene/methanol equal to 1/15 [33]. Thus, 2,2,3,3- tetrafluoropropyl alcohol is obtained in autoclave at proportion: tetrafluoroethylene/methyl alcohol (1/15) in the presence of CaCO3 and di-tert-butylperoxide at 125 oC and pressure 0.8 bar during 6 hours [33,36]. It is stated [8,29,41], that hexafluoropropylene and thermal perfluorolefines RCF=CF2 (R = CF3, C1-C4-perfluoralkyl) react with methyl alcohol in the presence of dialkylperoxides [41], producing respective partly fluorinated alcohols. It is stated [34], that telomeric alcohols H(CFRCF2)nCH2OH (R = F, CF3, n = 1; R = F, n = 2) obtained out of tetrafluoroethylene and hexafluoropropylene and methyl alcohol in the presence of di-tert-butylperoxide better produce under laser acceleration, UV-irradiation [35] or in the presence of base (for example, NaOCH3) [34]
This information shows the general interest to developing of such type alcohols obtaining
technologies, which availability essentially widens the sphere of practical application.
At the same time the attention should be paid to mechanism of this process. It is generally
accepted, that it includes generation of radical or anion radical under the action of
initiator on alcohol, which leads the process [18-21]. It can be supposed, that present
reactions as in the case of tetrafluoroethylene reaction with methyl alcohol in the presence
of tert-butyl peroxide proceed through generation of intermediate radicals, generated
out of alcohols by removal of hydrogen radical from  -position
under the action of tert-butyl peroxide with further interaction of these radicals with
tetrafluoroethylene, producing oligomerization products. At using of tert-butyl peroxide
excess partly fluorinated alcohols and very small amounts of tetrafluorethylene oligimerization
products are obtained.
-position
under the action of tert-butyl peroxide with further interaction of these radicals with
tetrafluoroethylene, producing oligomerization products. At using of tert-butyl peroxide
excess partly fluorinated alcohols and very small amounts of tetrafluorethylene oligimerization
products are obtained.
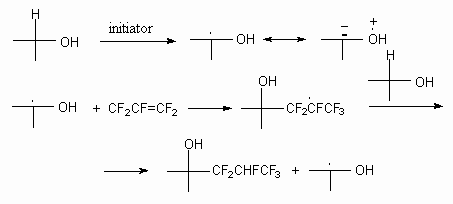
The reaction mechanism includes photochemical stimulation of fluoroolefine's double bound and its reaction with alcohol, resulting in generation of alkoxy-radical. Further reaction of last one mentioned with fluorolefine produces radical, which eliminate hydrogen radical from alcohol with formation of reaction product [42].
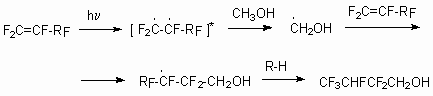
In the pilot variant there had been realized safe and wasteless production of partly fluorinated alcohols on the base of tetrafluoroethylerne and hexafluoroethylene and alcohols reactions in the presence of peroxide initiator with yield close to quantitive. It is stated, that chain length in partly fluorinated alcohols depends greatly on proportion of tetrafluoroethylene to initiator, that allows to obtain the telomeric alcohol needed. On the basis of obtained results the telomeric alcohols obtaining commercial plant was created, that really extended the possible producing methods of semi-products for fluoroorganic synthesis. The production of partly fluorinated carboxylic acids and dialkyl ethers is developed on the base of telomeric alcohols.
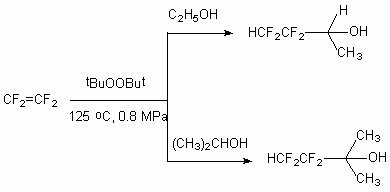
The syntheses of partly fluorinated secondary and tertiary alcohols out of tetrfluoroethylene and hexafluoropropylene [8] were carried out under following conditions: tetrafluoroethylene reacts with ethanol or iso-propanol at 125oC, pressure 0,8 MPa and excess of tert-butyl peroxide. At these conditions 3,3,4,4-tetrafluorobutan-2-ol and 3,3,4,4-tetrafluoro-2-methylbutan-2-ol are formed with high yield. A small amount of tetrafluoroethylene telomerization products is also formed as by-products, they are extracted using simple distillation [43].
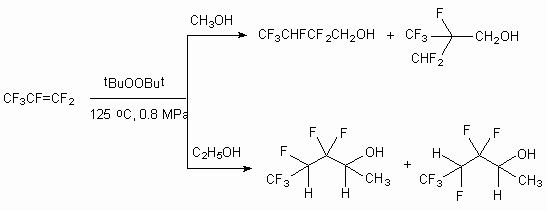
In case of hexafluoropropylene reaction with methyl and ethyl alcohols in the presence of tert-butyl peroxide only 2,2,3,4,4,4-hexafluorobutan-1-ol and 3,3,4,5,5,5-hexafluoropentan-2-ol are formed respectively. The opportunity of two radicals generation out of hexafluoropropylene according to assymentric double bound supposes the formation of two isomeric alcohols. In fact, at interaction of hexafluoropropylene and methyl alcohol the formation of two alcohols and 2-difluoromethyl-2,3,3,3-tetrafluoropropan-1-ol (ratio 93:5) is recorded, while the reaction with ethyl alcohol produces mixture consisting of two stable conformers (ratio 58:38) [43].
If chlorotrifluoroethylene is introduced into the reaction and 2,5-bis-(tert-butylperoxy)-2,5-dimethylhexane is used as radical initiator [44], that HClFCCF2CH2OH is obtained as main product with 95% selectivity.
UV-irradiation can be an initiator of these processes, at that the products of alcohols regioselective addition according to multiply bond of fluoroolefine are formed [45]. Both hexafluoropropylene and perfluorinated alkyl vinyl ethers react in such reactions [46]. As a rule, two regioisomeric adducts are formed, the conversion of fluoroolefine is rather high.
Radiolysis of internal perfluoroolefines by 60Co  -irradiation
in alcohol media results in formation of secondary alcohols [46]. Thus, perfluor-2-butene
with methanol produces mixtures of threo/erithro in proportion of 1 to 1, while
in case of perfluor-2-pentene with methyl alcohol reactions two isomeric products are
formed in proportion 3:2. At the same time trans-perfluoro-4-methyl-2-pentene with methyl,
isopropyl and ethyl alcohols produces only two diastereoisomers.
-irradiation
in alcohol media results in formation of secondary alcohols [46]. Thus, perfluor-2-butene
with methanol produces mixtures of threo/erithro in proportion of 1 to 1, while
in case of perfluor-2-pentene with methyl alcohol reactions two isomeric products are
formed in proportion 3:2. At the same time trans-perfluoro-4-methyl-2-pentene with methyl,
isopropyl and ethyl alcohols produces only two diastereoisomers.
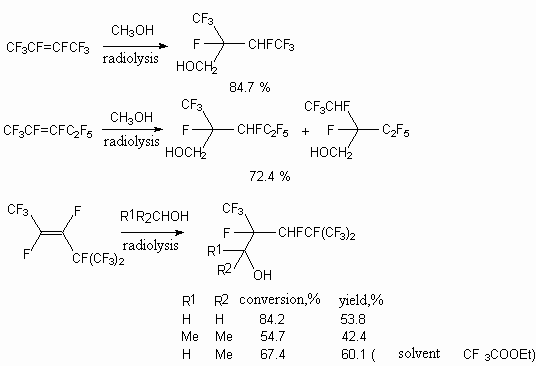
The free radical addition of alcohols, initiated by  -irradiation
also exists for non fluorinated cyclic olefins, at that the mixture of diastereoisomers
is formed [47,48].
-irradiation
also exists for non fluorinated cyclic olefins, at that the mixture of diastereoisomers
is formed [47,48].
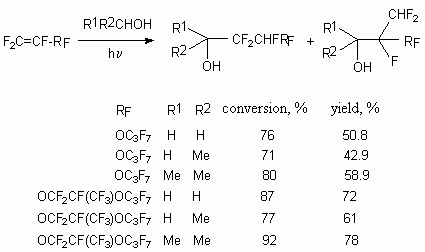
The cyclic alcohols also regioselectively reacts with hexafluoropropylene under the influence
of  -irradiation
or in the presence of di-tert-butyl peroxide with formation of the replacement
product of hydrogen atom at tertiary carbon. (table. 1) [49].
-irradiation
or in the presence of di-tert-butyl peroxide with formation of the replacement
product of hydrogen atom at tertiary carbon. (table. 1) [49].
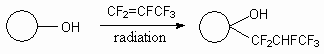
Table 1. Free radical addition of alcohols to hexafluoropropylene [49].
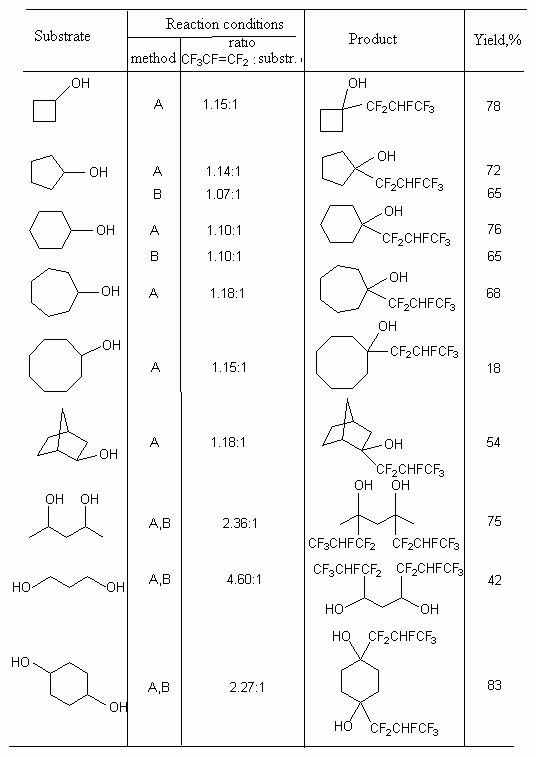
A -  -irradiation,
20 oC, 10 days: B - di-tert-butyl peroxide, 140 oC, 24
hours.
-irradiation,
20 oC, 10 days: B - di-tert-butyl peroxide, 140 oC, 24
hours.
It should be noted, that for some alcohols the formation of dimeric products exists but in small amount. Thus, for cyclohexanol and exo-bisyclo[2,2,1]-heptan-2-ol the yield of dimeric product was 40 and 11% respectively.
In case of hexafluoropropylene reactions with different cyclic and acyclic diols in these conditions the replacement of hydrogen atom according to both tertiary carbon atoms with CF2CHFCF2 group takes place (table 1) [49].
It is not necessary to use alcohols for these purposes, you can base yourself on dialkyl
ethers. Thus, the authors of the work [50] showed, that 1,2-dimethoxyethane, 1,2,-diethoxyethane,
1,5-diethoxyethyl ether and 2-methoxyethanol in the presence of crown ether (18-crown-6)
at 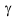 -irradiation
treatment react with hexafluoropropylene, producing replacement products of methoxyl group
hydrogen with 2-H-hexafluoropropenyl group.
-irradiation
treatment react with hexafluoropropylene, producing replacement products of methoxyl group
hydrogen with 2-H-hexafluoropropenyl group.
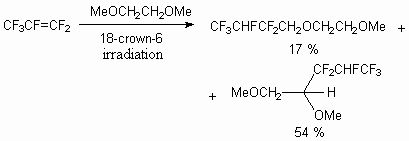
Partly fluorinated alcohols are also obtained by reduction of respective esters of perfluorcarboxylic acids [51].
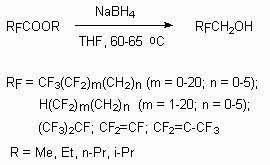
For example, methyl ether of perfluorooctanoic acid produces CF3(CF2)6CH2OH with the yield of 98.3% at 100% alcohol conversion.
If you use oxidizing agents, for example oxygen or fluorine derivatives in the presence of Al2O3 or copper powder, then instead of perfluoroolefine you can use partly fluorinated hydrocarbons. Thus, the oxidization of 1,1,1-trifluoroethane at these conditions results in formation of CF3CH2OH [52].
The fluorinated alcohols like RFCH2OH (RF = C 1-10 perfluoroalkyl) are obtained with high yield and selectivity by fluoroalkylhalides RFCH2X (X = halide) condensation with carboxylated salts like HA( CR1R2)mCOOM (A = O, NH, M = alkali element, m = 3-5) at 100-180 oC , pressure 0.1 - 2 bar in 1,3-dimethyl-2-imidazolidone medium [53].
The process of Grignard reagent CF3CF2CF2MgI and silicon containing aldehydes interaction is more complicated, resulting in formation of general formula R3Si(CH2)nCH(OH)RF (n = 1-3) alcohols [54] .
Unsaturated alcohols like RFCH=CHCH2OH (RF = perfluoroalkyl, polyfluoroalkyl) are obtained by reaction of RFCF2CH=CH2 with water in the presence of acidic catalyst [55]. Analogously CF3CF2(CF2CF2)2CH=CH2 by water action in the presence of zeolite, containing silicon, at 300 oC is turned into alcohol CF3(CF2)4CF=CHCH2OH with yield of 80 % [55].
Fluorine Notes, 2003, 31, 1-2
