Fluorine Notes, 2003, 31, 3-4
Innovation in the synthesis of perfluorinated organics through electrochemical fluorination in anhydrous liquid hydrogen fluoride: peculiarities of the method and applications of perfluorinated organics
V.A. Matalin, G.I. Kaurova, D.D. Moldavskiy, V.G. Barabanov, O.V. Blinova
Federal State Unitary Enterprise «Russian Scientific Center
"Applied
Chemistry",
St.Petersburg, Russia
(Financially supported by USA and performed under the contract to
the International Science and Technology Center (ISTC), Moscow.)
Electrochemical fluorination (hereinafter: ECF) is a method to introduce
fluorine into organic or inorganic substrate through some electrode reactions.
The ECF method involves passing of a direct current through a solution of some
initial organic substance in anhydrous hydrogen fluoride.
J.Simons (USA) developed the most part the ECF method in early 1940th [1].
ECF provides replacement of all hydrogens by fluorines, saturation of triple or
double C=C bonds and aromatic systems with fluorine, and fragmentation of carbon
skeletons, while functional groups and heteroatoms available in the original
molecule stay untouched.
ECF demonstrates a number of advantages over other methods of synthesis of
perfluorinated organic substances:
- Hydrogen fluoride serves as the source of fluorine. This obviates the otherwise necessary stage of fluorine manufacture using hydrogen fluoride for the raw material.
- The needed equipment is relatively simple because the required electrolytic cell (electrolyzer) is a single chamber apparatus.
- In spite of inevitable destruction of the initial substance ECF allows conserving most of the functional groups or heteroatoms (N, O, S) available in the initial molecule.
Among numerous perfluorinated substances produced by ECF there are perfluorinated tertiary amines, perfluoroethers and perfluorocarbons. Those substances are of prime interest for industry being inert, environmental friendly, non-flammable and fire-safe liquids applicable as heat-carriers or liquid dielectrics in a number of technical applications in wide temperature range. The derivatives of fluoroanhydrides of carbonic and sulfonic acids are efficient surfactants needed as usual materials in transportation and metallurgy. The derivatives of perfluoromethyl- or perfluoroethyl- sulfonic acids are very promising raw materials for electrolyte in the new generation of chemical current sources [2-4].
On the other hand, ECF has some disadvantages that may limit its usage. One important disadvantage is destructive fluorination of the initial substance that occurs simultaneously with the synthesis of a perfluorinated target molecule. It usually results in a mixed product, while the yield of the target substance may happen to be rather small.
The share of the current spent in the side reactions of destructive fluorination grows with the number of carbons in the initial molecule. Fluorination of molecules containing 8-9 or more carbons often results in gums that tender to accumulate in the electrolyte and to stick to the electrodes thus hindering the use of continuous electrolysis process.
In our work we found some routs to remedy the above-mentioned problems or, at
least, to decrease considerably their influence on the ECF process.
In our study there were two different lines of inquiry.
The first avenue of our investigation concerned with substances that being
dissolved in liquid hydrogen fluoride do not conduct electric current at all, or
the electrolyte life-time is very short during their ECF, gumming is very
strong, and the yield of the target substance is very low. The results of the
study are presented below in Chapter 1.
The second avenue of investigation was rather traditional, though it required
some technological innovations. It was suggested to isolate not only the main
product, but all by-products formed in considerable amounts. This idea was
tested with ECF of n-tributylamine (C4H9)3N. The results of the study are
presented below in Chapter 2.
I. COMBINED FLUORINATION
The first avenue of our investigation concerned such substances that being dissolved in liquid HF do not conduct electric current or their ECF is followed with strong gumming of electrolyte, reducing its lifetime considerably.
In such cases they usually add NaF or some other alkali metal fluoride to improve ECF [5]. However, adding of alkali metal fluorides accelerates corrosion of nickel anodes and does not prevent gumming of electrolyte.
To inhibit the electrolyte gumming they often use such electrolytic additions as n-butylmercaptan or some other molecules with S-H or S-S bond [6].
The main inconvenience of such additions is their obnoxious pungent odour with low threshold of sensitivity. It hampers their usage in industry for environmental reasons. It also should be noted that mercaptans undergo electrochemical fluorination simultaneously with the main initial raw material and therefore a major portion of electricity is consumed in the formation of their perfluorinated derivatives.
In this our work we tried to improve the ECF of perfluorinated organics through
selection of such electrolytic additives that would lead to tertiary
perfluoroamines (substances of both industrial and commercial interest),
suppress gumming of electrolyte and increase its lifetime.
We tested tertiary amines for electrolytic additives in electrochemical
fluorination of various classes of organic substances. We studied ECF of
alkylsulfuryl fluorides CnH(2n+1)SO2F, ethers and other partly fluorinated
organic substances, sparingly soluble in HF or making solutions that do not
conduct electric current, e.g., benzotrifluoride. It is obvious that the yield
of the target product grows considerably (2-3 times) if partly fluorinated
organic substances (PFOS) are used for initial raw materials in ECF. However,
their application by now was limited because the most of them are insoluble or
do not conduct electric current when dissolved in HF. Their solubility in HF
grows considerably in triple systems (hydrogen fluoride - amine - PFOS).
From the composition of ECF products one may deduce that there was combined
fluorination of organic substances belonging to two different classes.
Experiment (experimental technique)
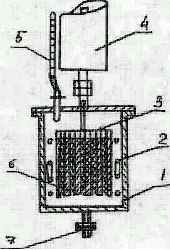
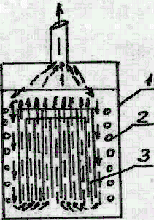
In our tests we used a carbon steel Simons electrolyzer of volume 0.66l (see Fig.1). To recycle HF carried with electrolysis gases the electrolyzer (1) was equipped with a reflux condenser (4). A coiled tube (2) with circulating water was provided in the electrolyzer to cool electrolyte. The interleaved anodes and cathodes were made of nickel plates and formed a package (3).
|
|
|
| Fig. 1.
Electrolyzer for electrochemical fluorination 1 - electrolyzer housing; 2 - cooling coiled tube; 3 - electrode package; 4 - reflux condenser; 5 - feed line for organic substrate; 6 - fluoroplast-4 gaskets; 7 - discharge valve. |
|
In our experiments the current load was constant (15 A). The current density in
all tests was also constant (0.03A/cm2). The efficient surface of the anodes was
equal to that of the cathodes (504 cm2).
We conducted electrolysis of the solution that contained 5-15% by weight of an organic substance, and 5-15% by weight of a tertiary amine in liquid hydrogen fluoride. The organic blend was being fed periodically (once or twice per hour) or was used without adding.
The density of the crude product (liquid fluorination products) exceeded considerably the density of the electrolyte, therefore the product tended to accumulate at the bottom of our electrolyzer, from where we decanted it under water in equal time intervals and neutralised with soda. Then we treated the crude product with water-alcohol solution of potassium hydroxide and dried with silica gel.
Dry crude product free from HF and non-fluorinated impurities was then distilled
to give the target product of purity 98.0-99.5%. The product was analysed by
gas-liquid chromatography (GLC) with the help of «Zvet-100» GLC (Russia)
equipped with a katharometer and a column packed with silochrom-80 with 20% of
![]() ,
,![]() ,
,![]() -trisbetacyanacetophenone.
-trisbetacyanacetophenone.
After passing of predetermined amounts of electricity (200Ah/l) we decanted crude product of electrolysis. At the same time we determined the yield-by-current of our crude product and analysed it with the help of GLC. The electrolyte was then analysed for the content of hydrogen fluoride and tertiary amine. The electrolysis gas was also analysed at GLC. Each time we found that the anode gas contained CF4, C2F6 and other products of destructive fluorination, F2O was also revealed (in small quantity) due to fluorination of water traces present in HF or in the initial raw materials. Hydrogen was formed in the cathode reaction.
Experimental results and discussion
1.1 ECF of alkylsulfurylfluorides
Combined fluorination of tertiary amines and alkylsulfurylfluorides was studied
using as for an example the preparation of perfluorooctanesulfurylfluoride that
has got multiple applications in industry. For instance, it is used for raw
material in surfactant production.
The main anode reactions are:
![]()
![]()
Here: RH= C2H5, C3H7, C3H5, C4H9, or: (RH)3N
= C5H5N,
RF = C2F5, C3F7, C4F9 or: (RF)3N
= C5F11N,
There are a number of methods for the manufacture of C8F17SO2F:
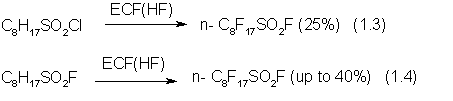
At the early stage the yield of the ECF process according to (1.3) is about 25%,
but very soon it drops considerably, possibly, due to the participation of
chlorine [7].
In the case of ECF according to (1.4) some electrolytic additive is necessary because the solution of C8H17SO2F in hydrogen fluoride does not conduct electric current. Sodium fluoride or some mercaptan (mostly n-butylmercaptan) is usually applied for such additives. The disadvantages of their addition are already discussed above.
We studied ECF of C8H17SO2F using for electrolytic additives some tertiary amines (e.g., (C2H5)3N, (C3H5)3N, (C3H7)3N, (C4H9)3N, C5H5N). The experimental results are shown in Table 1.
Table 1. Electrochemical synthesis of C8F17SO2F in various HF-based electrolytes
|
Initial raw material |
Anode current density, |
Quantity of passed electricity, |
Current efficiency, % |
Main components of crude product, |
|||
|
Ah |
Ah/litre |
C8F17SO2F |
C8F18 |
(RF)3N* |
|||
| C8H17SO2Cl [5] | < 0,002 | - | - | 25 | - | - | - |
| C8H17SO2F (+NaF,C4H9SH)[5] | < 0,002 | up to 40 | - | - | - | ||
| C8H17SO2F + (C2H5)3N | 0,03 | 975 | 1477 | 37 | 42 | 17 | 26 |
| C8H17SO2F + (C3H5)3N | 0,03 | 1584 | 2400 | 52 | 50 | 14 | 24 |
| C8H17SO2F + (C3H7)3N | 0,03 | 1133 | 1718 | 46 | 57 | 20 | 12 |
| C8H17SO2F + (C4H9)3N | 0,03 | 984 | 1490 | 36 | 45 | 12 | 25 |
| C8H17SO2F + C5H5N | 0,03 | 390 | 590,5 | 58 | 15** | 10 | 55** |
* percentage of the main perfluorinated product; in the case of C5H5N the main
product is perfluoropentane C5F12;
**reduced content of C8F17SO2F and elevated content of the fluorinated product may be due to dissolving of C8F17SO2F in C5F12.
Rather quick drop of the current efficiency is a serious disadvantage of the available methods for C8F17SO2F manufacture. In full-scale production it may cause considerable rise of the product cost.
Our experiments proved that the addition of tertiary amines allows to decrease gumming and to prolong electrolysis with sufficiently high current efficiency. In this case among the electrolysis by-products there are perfluorocarbons and perfluorinated amines, both of considerable industrial demand as unique dielectric liquids, heat-carriers, solvents, etc.
From published data it is known that electrochemical synthesis of C8H17SO2F is usually conducted at very low current densities (0.001-0.002A/cm2 ) [2], having in mind to reduce the rate of the initial organic substance destructive fluorination. The addition of tertiary amines made it possible to increase the work current density to 0.03A/cm2 .
1.2. ECF of partly fluorinated substances
ECF of partly fluorinated substances results in much higher yield of the target product than that of their hydrocarbon analogues. It provides them good prospects in the future usage. However, partly fluorinated organics is often insoluble in hydrogen fluoride and does not conduct electric current. The problem is solved through adding of tertiary amines. As an example one may consider ECF of (C6H13)2NC6F11, C2F4HSO2F, or C6H5CF3. Thus, e.g., the main anode reactions that occur during ECF of C6H5CF3 with addition of triallylamine are as follows:
![]()
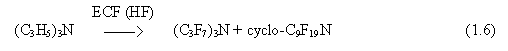
In our experiment the mole ratio (C6H5CF3 : triallylamine) = 1:1, and the total
concentration of organics in HF was 22% by weight. The experiment resulted in
127g of the crude product, the current efficiency being 78%. The results are
presented in Table 2
Table 2.ECF of C6H5CF3 and (C3H5)3N
|
Initial raw material |
Anode |
Quantity of passed electricity |
Current efficiency |
Main components of crude product, |
||
|
A/cm2 |
Ah |
Ah/litre |
% |
C6F11CF3 |
(C3F7)3N+cyclo-C9F19N |
|
| C6H5CF3 |
Poorly soluble in HF and does not conduct current |
|||||
| C6H5CF3 + (C3H5)3N | 0,03 | 300 | 454,5 | 78 | 57 | 20 |
From the above data it is obvious that the proposed method allows improving of ECF indices when it concerns the synthesis of various classes of perfluorinated organics.
The advantages of the method are as follows:
1. Various tertiary amines may serve as electrolytic additives, thus providing
ECF of poorly soluble organics and/or substances that form non-conductive
solutions.
2. The method performance is improved as the presence of tertiary amines effects
the desired decrease in the ECF electrolyte gumming. The ECF process is long
enough and results in rather high yields of the target products.
3. The ECF by-products are tertiary perfluoroamines that may have various
industrial applications due to their unique thermophysical and dielectric
properties.
4. The process is flexible as it concerns the materials. Any tertiary amine can
be applied for such electroconductive additives. Reasoning from the current
demand in a tertiary perfluoroamine one may choose the appropriate additive.
II. ELECTROCHEMICAL FLUORINATION OF TRIBUTYLAMINE.
ECF of n-tributylamine in anhydrous liquid hydrogen fluoride occurs according to the equation as follows:
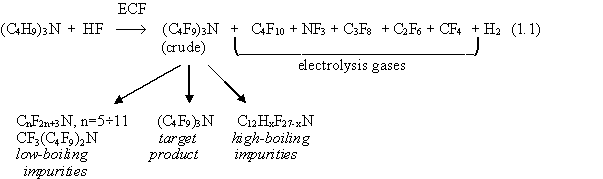
From the reaction equation one may notice that in electrochemical fluorination of tributylamine (TBA) a number of fluorinated by-products are formed along with perfluorotributylamine.
Fluorocarbons were reported [2-6] to be usual gaseous by-products in the synthesis of perfluorotributylamine (or some other tertiary fluoroamines) formed due to destructive fluorination in amounts up to 0.5-1.0 kg per 1kg of the target product. Their isolation is rather difficult as they are inevitably mixed with hydrogen (the main cathode reaction product), the percentage of the latter being 80-85%.
Electrolysis gas released during electrolysis of TBA contains hydrogen and some low-boiling products of TBA destructive fluorination: CF4; NF3; C2F6; C3F8; C4F10. The content of C3F8 is about 30%, that of C4F10 may reach 45% by weight.
C3F8 and C4F10 are efficient dielectric heat-carriers that find use in various
branches of industry.
The absorption method designed for their isolation from electrolysis gases uses
the main product, i.e. perfluorotributylamine (PFTBA), for the absorbent.
The parameters of the laboratory absorption column were as follows: diameter 30mm; height 2 m; Levin spiral-prismatic packing sized 3X3mm. The column was sprayed with perfluorotributylamine cooled to 00C.
In the process of absorption the solvent was absorbing perfluorobutane along with main perfluorinated or polyfluorinated impurities; and hydrogen, NF3, etc. were being released into the atmosphere from the column top.
The saturated solvent (perfluorotributylamine) entered an evaporator where it
was heated to 900C to provide desorption of the dissolved gases.
At those conditions the degree of octafluoropropane trapping was about 80%, and
that of perfluorobutane was 99%.
Therefore, the method allows isolating from ECF electrolysis gas not only tributylamine, but also perfluorobutane (C4F10) and octafluoropropane (C3F8), both having industrial applications.
We found that the low-boiling fraction of our electrolysis liquid product (crude
PTBA) contained perfluorohexane C6F14, linear amines [CF3N(C4F9)2, (C3F7)3N,
(C2F5)N(C4F9)2] and cyclic amines (C9). By their physico-chemical properties all
those components are classified with inert dielectric liquids and may find
appropriate employment. However, their boiling points are so close that their
individual isolation is very laborious and would only be reasonable in
full-scale PFTBA manufacture. It is not so difficult to separate them as a
product blend (BLEND -I). According to our measurements the BLEND -I dielectric
properties are not worse than those of its individual components are. Besides of
that, we can easily separate another PFTBA-based blend (BLEND-II) with the ratio
"PFTBA : (BLEND -I)" = about (85: 15). The BLEND-II has dielectric properties
similar to those of PFTBA, and its freezing point is even much lower (minus 800C
instead of minus 550C) making it applicable in even wider temperature range. Of
course, the yield of BLEND-II would be higher and the cost lower, than those of
pure PFTBA. We think that the BLEND-II may be attractive for many applications
in industry.
Therefore, the developed combined technology suggests isolation not only of the
main (target) perfluorotributylamine but also the process by-products formed in
considerable amounts. We think that it may serve for a basis of a universal
technology for electrochemical synthesis of perfluorinated organic products.
CONCLUSION
We are offering an efficient ECF method that allows fluorinating of various
organic substances, even poorly soluble or forming non-conductive solutions with
HF, through the usage of tertiary amines for electrolytic additives.
In so doing perfluorinated organics belonging to various chemical classes is
produced with rather high yield. Those perfluorinated substances are promising
surfactants, dielectric heat-carriers, materials for chemical current sources,
etc.
Using ECF of n-tributylamine (C4H9)3N as an example we realised the isolation
not only of perfluorotributylamine, that was the main reaction product, but also
of all process by-products formed in detectable amounts.
The combined application of the techniques developed during the progress of this
our study may provide the basis for a universal technology of electrochemical
synthesis of perfluorinated organic products.
We have got a patent for the new synthesis method for perfluorinated organic
substances belonging to various chemical classes through ECF.
List of references:
1. Fluorine chemistry Ed. By J.H.Simons, N.Y., 1950.
2. "Organofluoric chemicals and their industrial applications", ed. R. E. Banks, Horwood: Chichester, 1979, p.45.
3. S. Nagase "Fluorine Chemistry Reviews" ed. P. Tarrant, Marcel Dekker: New
York, 1967, p. 77; S. Nagase, T. Abe and H. Baba, Bull. Chem. Soc. Jpn., 1968,
41, 1921.
4. R.E. Banks, Fluorocarbons and Their Derivatives, Macdonald: London, 1970 (2nd
edn.).
5. The Role of adsorption in electrochemical perfluorination of
alkylsulfofluorides, G. Cauquis, B.Keita and G.Pierre. J. Electroanal. Chem.,
100 (1979) 205 ? 215.
6. R.D. Danielson and J.W. Sargent, Pat. USA 3028321, C25B 3/08, issued in 1962.
7. T. Gramstad, R. N. Haszeldine. J. Chem. Soc., 1956, 173 (1973); T. Gramstad,
R. N. Haszeldine, ibid, 1957, 2640.
8. Simons J. H., J. Electrochem. Soc., 1949, 95, p.47; Simons J. H. and other,
ibid. 1949, 95, p.53; p. 55, p. 59, p. 64; Simons J. H., US Pat. 2 519 983/1950
(3M).
9. J. H. Simons and T. J. Brice, "Fluorine Chemistry", ed. Simons J. H.,
Academic Press: New York, 1954
10. J. Burdon and J. C. Tatlow, "Advances in Fluorine Chemistry", ed. M. Stacey,
J.C. Tatlow and A. G. Sharpe, Butterworths: London, 1960, Vol. 1, p. 129
11. I.L.Serushkin, G.A. Tedoradze, G.I.Kaurova, "Electrochemistry (Russian)",
1975, 11, issue 5; 1976, 12, issue 1, 3, 4.
12. I.N. Rojkov, "Anode fluorination" in "Organic Electrochemistry. An
Introduction and a Guide", Edited by M.M.Baizer and H. Lund, N.Y.: Marcel Dekker,
1983; D. Lines and H. Sutcliffe, J. Fluorine Chem., 1981, 17, 123.
13. A.J. Rudge, " Industrial Electrochemical Processes", ed. A.D. Kuhn, Elsevier:
Amsterdam, 1971, p. 77.
14. E.A. Kauck and J.H.Simons, US Pat. 2 644 823/1953.
15. N. Watanabe, Denki Kagaku, 1968, 36,172.
Federal State Unitary Enterprise "Russian Scientific Center "Applied
Chemistry",
St.Petersburg, Russia Dobrolubov ave, 14, St. Petersburg, 197198, Russia
Tel/Fax: +7-(812)-103-05-84;
E-mail: rscac@mail.wplus.net; kaurova@mail.wplus.net
Financially supported by USA and performed under the contract to
the International Science and Technology Center (ISTC), Moscow,
Luganskaya ulitsa, 9, 115516, Moscow
Internet: http://www.istc.ru
Fluorine Notes, 2003, 31, 3-4