Fluorine Notes, 2003, 30, 3-4
THE USE OF TETRAFLUORETHYLENE AND HEXAFLUORPROPYLENE IN THE SYNTHESIS OF PARTLY FLUORINATED ALCOHOLS AND DIALKYL ETHERS
A.A.Ilyin *, U.L. Bahmutov.*, A.N. Ilyin.*, L.M. Ivanova*, G.G. Furin **, T.G. Tolstikova **
* JS Galogen, 614113, Russia, Perm city, ul. Lasvinskaya,
98; E-mail : lexaaa@yandex.ru
** Novosibirsk Institute of organic chemistry named after N.N.Vorozhtsov
Siberian branch of the Academy of Science of Russian Federataion
Abstract
Here approaches to synthesis of partly fluorinated alcohols on the basis of tetrafluorethylene and hexafluorpropylene are analyzed. The interaction of ethyl and isopropyl alcohols together with tetrafluorethylene in the presence of tert-butyl peroxide results in formation of 3,3,4,,4-tertafluorobutan-2-ol, and the interaction of methyl alcohol and hexafluorpropylene at these conditions results only in formation of 2,2,3,4,4,4-hexafluorobutan-1-ol, while the one of ethyl alcohol results in formation of mixture of conformers of 3,3,4,5,5,5-hexafluoropentan-2-ol in proportion about 3:2. The interaction of partly fluorinated alcohols with freon 22, hexafluoropropylene, formaldehyde, pentafluorochlorobenzene in the presence of KOH and concentrated sulphuric acid was investigated. The opportunity of obtaining of dialkyl ethers using commercially available partly fluorinated alcohols was considered.
The interaction of hexafluoropropylene and methyl (ethyl, isopropyl, 2,2,3,3-tetrafluoropropyl) alcohols or 2,2,3,4,4-hexafluorobutan-1-ol in the presence of KOH results in formation of partly fluorinated dialkyl ethers due to addition of alcohols to double bond of hexafluoropropylene. The alkyl esters of 2,2,3,4,4-hexafluoropropionic acid were produced when dialkyl ethers were treated by concentrated sulphuric acid, while alkylpentafluoropropenyl ether was produced when KOH was used for treatment.
The new experimental data concerning using of telomeric alcohols reactions with unsaturated and hetero-organic compounds were discussed. The processes of oxidation and reactions of telomeric alcohols in strongly acid medium was analyzed . The toxicity of partly fluorinated alcohols and their derivatives was discussed too.
The chemistry of fluorine compounds is intensively developing due to techniques needs of new fluorocontaining materials, possessing specific properties. The expanding of this class compounds practical using field is determined not only by the level of fundamental research but to a greater extent by availability of source materials and by their level of technological developmental work. The most available and extensively used semi-products are tetrafluoroethylene and hexafluoropropylene, which are raw material for synthesis of many fluorine materials. Planning the obtaining of materials needed the opportunity of these perfluorolefines treatment with different reagents should be used, taking into account the main mechanisms of their reactivity.
The aim to approach the solving of two problems on the basis of available and relatively cheap tetrafluoroethylene and hexafluoropropylene was formulated in this work. At first to find alternative substitute for Freon 113, which was taken out of production because of its high ozone activity, and to suggest new solvents with wide fields of applications. Secondly to develop technologically acceptable synthesis of partly fluorinated secondary and tertiary alcohols on the basis of tetrafluorethylene and and hexafluorpropylene and to show synthetic opportunities of their involving into different fluororganic synthesis reactions, especially for fluorinated dialkyl ethers synthesis, and also to show the opportunity of application of such compounds as ozone-friendly solvents and semi-products.
The problems of creating the new ozone friendly solvents is to great extent determined by opportunity of freon 113 replacement, which is extensively used as solvent. The main drawback of this freon is limited and low solubility of hydrocarbon oils in it and insolubility of fluoroorganic and silicon organic liquids in it. Considerable efforts made to solve this problem didn't yet have a success. As one of the alternatives the authors of work [1] suggested hexafluorocyclobutane, which method of synthesis out dichlorohexafluorocyclobutane (the last mentioned is obtained using dimerization of trifluorochloroetylene) makes it commercially affordable and effective solvent for different azeotropic compositions. At the same time partly fluorinated dialkyl ethers are of great interest for these purposes. 3M company has developed freon HFE 7110 (CH3OCF2CF2CF2CF3), though it also has a number of drawbacks, while tetrafluoroethyldifluoromethyl ether (CHF2CF2OCHF2) can replace freon 11 as a foaming agent for production of foamed plastic and during dry etching processes in microelectronics instead of perfluorocarbones [2]. The ethers like C4F9OCH3 (BP 61 oC), (CF3)2CFCF2OC2H5 and others were used for extraction of essential oils of lavender and they had shown good results as for the quality of extractable substance and also for fineness of under formation oils, as carrier-solvents of surface active materials, coatings, agents for form release, oil-water repellents [4]. These ethers were used as solvents for chemical reactions, especially for synthesis of fluoropolyalkyl ethers amides, used for magnetic producing mediums [5], and oxidization of tetrafluorethylene [6].
The synthesis of partly fluorinated ethers is carried out by alcohols acting on hexafluoropropylene in the presence of alkali [7,8], alcoholates in toluol [9], dioxane [10] or methyl alcohol [11]. The interaction of hexafluorpropylene and polyoxaalkylenglycols in the presence of water traces produces products of addition by double olefin bond, which have found their application as compression, motor and vacuum oils [12]. Entirely fluorinated dialkyl ethers, for example (CF3)2CFCF2OCnF22n+1, obtained by reaction of octafluoroisobutylene with alcohols and by further additional electrochemical fluorination in anhydrous hydrogen fluoride, are used as solvents in microelectronics, as refrigerating and foaming agents, as medium for polymerization etc [13-15].
We have determined, that interaction of hexafluoropropylene with alcohols in the presence of catalytic quantities of sodium alcoholate or alkalies at temperatures 20-40 oC at passing of gaseous hexafluoropropylene through solution (or carrying out reaction in autoclave) results in formation of the addition products of alcohols to double bond. The reaction path to small extent depends on carrying out conditions. Fluorine replacement products at terminal multiple bond for alkoxy-groups and alkyl esters of 2,3,3,3-tetrafluoropropionic acid are formed as by-products. Their quantities do not exceed 5% (look through work [16]).
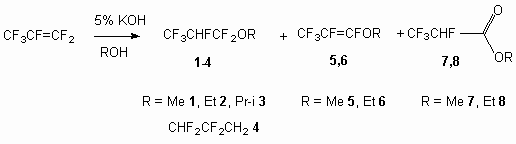
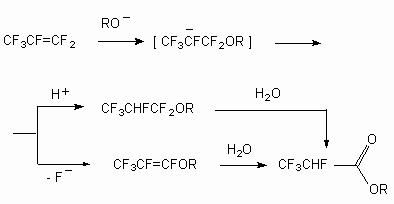
The reaction goes due to alkoxyl anion attack on carbon atom at double bond of hexafluoropropylene
with generation of intermediate C-anion. The last one reacts with proton of the system producing
main reaction product (compounds 1-4). The opportunity of carbanion stabilization
by means of fluoride-ion elimination from ![]() -position (fragment CF2) results in formation of replacement product
of fluorine atom at double bond (compounds 5, 6). Anhydrous hydrogen fluoride formed
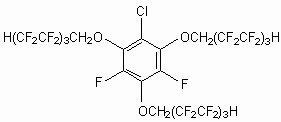
at that reacts with alkali, and the water formed participates in formation of addition product and
can carry out the hydrolysis as of compounds 5, 6 and also of compounds 1-4.
The fact of anhydrous hydrogen fluoride evolving from reaction mixture and formation of alkyl esters
of 2,3.3,3- tetrafluoropropionic acid (7, 8) denotes this.
-position (fragment CF2) results in formation of replacement product
of fluorine atom at double bond (compounds 5, 6). Anhydrous hydrogen fluoride formed
at that reacts with alkali, and the water formed participates in formation of addition product and
can carry out the hydrolysis as of compounds 5, 6 and also of compounds 1-4.
The fact of anhydrous hydrogen fluoride evolving from reaction mixture and formation of alkyl esters
of 2,3.3,3- tetrafluoropropionic acid (7, 8) denotes this.
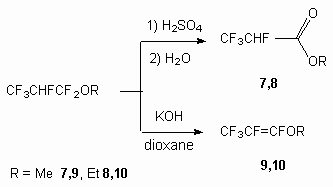
Indeed when heating the compounds 1 and 2 with concentrated sulphuric acid at the temperature of 40 oC during 1 hour and at further treatment of reaction mixture with water the formation of compounds 7 and 8 with quantity yield (see [7]) takes place, and heating with solid KOH in dioxane produces compounds 9 and 10.
The structure of molecules 1-10 is confirmed by NMR spectroscopy, which are interpreted taking into consideration the information available for compounds of the like type (table 1), IR and mass-spectroscopy.
Table 1. The data of 1H, 13C and 19F NMR spectra of new partly fluorinated dialkyl ethers
|
Compound |
NMR spectra 1H |
NMR spectra 13C |
NMR spectra 19F |
|
1 |
(H2) 4.72 (36.4; 9.2); (H4) 3.63; |
120.4 (C1, 1JCF 255.9; 2JCF 25.5; |
(F1) 88.4; AB-system (F3) 80.4 and 77.7 (JFF 151.6); (F2) -48.2 |
|
2 |
(H2) 4.71 (43.2; 5.5); (H4) 4.03 (7.1); |
120.3 (C1, 1JCF 280.0; 2JCF 25.4); 118.7 (C3, 1JCF 265.9; 2JCF 25.4); |
(F1) 87.5; AB-system 83.5 and 80.5 (JFF 153.4); (F2) -48.3 |
|
3 |
(H2) 4.66 (50); |
120.1 (C1, 1JCF 309.8; 2JCF 26.0); 118.7 (C3, 1JCF 277.3; 2JCF 21.9); |
(F4) 88.2; AB-system 85.9 and 82.1 (JFF 152.5); (F2) -48.1 (JFH 40; JFF 12.5) |
|
4 |
(H4) 4.29 t (50); |
119.9 (C1, 1JCF 281.4; 2JCF 27.6); 114.1 (C6, 1JCF 220.6; 2JCF 28.0); 109.1 (C3,5, 1JCF 250.1; 2JCF 35.8); 85.0 (C2, 1JCF 201.9; 2JCF 34.9); |
(F1) 88.4; AB-system (F5) 38.8 and (F6) 25.2; (JFF 149.7); AB-system (F3) 82. and 79.8 (JFF 148.9); (F2) -48.4 |
|
7 |
(H2) 5.27 (44.0; 6); (H4) 3.88; |
120.5 (C1, 1JCF 281.9; 2JCF 25.8); |
(F1) 87.6 (8; 12); (F2) - 41.2 (32; 10) |
|
8 |
5.10 (H2) ( 1JHF 45, 2JHF 7); 4.36 (H4) (2JHH 7.1); 1.34 (H5) (2JHH 7.1) |
120.5 (C1, 1JCF 281.9; 2JCF 27.8); |
(F1) 86.5 (8; 12); (F2) -42.2 (45; 10) |
Partly fluorinated alcohols like H(CF2CF2)nCH2OH (n = 1-6), which production is based on interaction of tetrafluoroethylene with methyl alcohol at high temperature and pressure in presence of peroxide initiator with decomposition point higher than 100 oC [17-19], have found a broad application for fluoroorganic synthesis [20]. Thus they are used for production of many fluorine-containing materials and they are semi-products for producing partly fluorinated carboxylic acids [21-25], alkanesulfinic [20] acids and fluorinated ethers of glycol [20]. At the same time in presence of basis they react as active O-nucleophiles, allowing to obtain the whole spectrum of ethers and esters [26]. The interest in development of definitely specified alcohol obtaining technologies had sharply increased in recent years, when the effectiveness of their using at production of information-reproducing medias, having the reproducing layer able to read information of laser, was shown and also when it was shown at production of high-performance surface active materials and photo-developing compounds in photography [27].
In new patents the fluoroalcohols obtaining method is based on the main process- radical addition of methyl alcohol to tetrafluoroethylene [18]. Mainly, the difference of patents is reduced to optimization of process carrying out conditions and intended for obtaining of key necessary alcohol 2,2,3,3-tetrafluoropropanol. It turned out [28-30], that the carrying out of the process is more effective in the presence of CaCO3. If at that the temperature of the process is maintained within 60-65 oC (4 hours), then 2,2,3,3-tetrafluoropropanol will be obtained [29], while at 125 oC and pressure equal to 0.8 bar (6 hours) you will get the mixture of alcohols [29]. The best results are obtained at the ratio of tetrafluoroethylene/methanol equal to 1/15 [30], when only 2,2,3,3-tetrafluoropropanol is obtained. It is stated [18,27,31], that hexafluoropropylene and terminal perfluoroolefines RCF=CF2 (R = CF3, C1-C4 - perfluoroalkyl) react with methyl alcohol in the presence of dialkylperoxides [31], producing corresponding partly fluorinated alcohols.
This information allows starting the common interest to development of such alcohols obtaining technologies, the availability of such alcohols greatly extends the field of their practical application.
The development of telomeric alcohols [H(CF2CF2)nCH2OH (n = 1-6)] production technologies [17,18], which based on reaction of tetrafluoroethylene and hexafluoropropylene with methanol in presence of peroxide initiator, stimulated of their commercial manufacture. They have found a wide application for fluoroorganic synthesis acting as syntones for introduction of partly fluorinated fragment and formation of dialkyl ethers. We think, that according to widening of their production influence the present methodology has great advantages and allows to get different polyfluorinated compounds with high economy. .
In the development of this approach the present article describe the opportunity of widening the field of application of available partly fluorinated alcohols for partly fluorinated dialkyl ethers and other objects with ether group synthesis. Freon 22, hexafluoropropylene, pentafluorochlorobenzene and formaldehyde were used as substrates with active haloid atoms or having high electrophilicity.
The synthesis of telomeric alcohols used in work was hold using method [18], based on production technology. At that it should be noted, that carrying out conditions of this process are important and their production technologies should be strictly followed. Thus, we have found, that at H(CF2CF2)2CH2OH 11 alcohol obtaining the formation of isomeric to it alcohol 12 was observed.
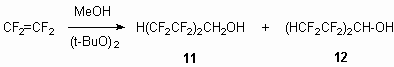
This result can be accounted for the fact, that in these conditions intermediate 2,2,3,3-tetrafluoropropyl alcohol 13 formed due to chain interruption performs as methyl alcohol and serves as source of radical 13a. The reaction scheme as follows:
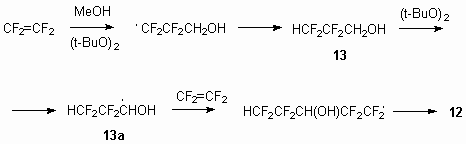
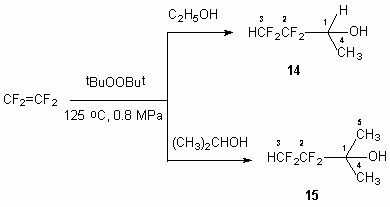
In development of previous researches [18] we have stated, that at carrying out the reaction of tetrafluoroethylene with ethyl and isopropyl alcohols in excess of tert-butyl peroxide at 125 oC and pressure equal to 0.8 MPa 3,3,4,4-tetrafluorobutan-2-ol 14 and 3,3,4,4-tetrafluoro-2-methylbutan-2-ol 15 are easily formed. The products of tetrafluoroethylene telomerization are formed as minor by-products, which are easily isolated by distillation.
It can be supposed, that these reactions as reactions of tetrafluoroethylene with methyl alcohol
in the presence of tert-butyl peroxide proceed through generation of intermediate radicals,
obtained from alcohols by elimination of hydrogen atom from ![]() - position under tert-butyl peroxide action with further interaction
of these radicals with tetrafluorethylene, producing oligomerisation products.
In case of using the excess of tert-butyl peroxide compounds 14 and
15 are obtained and also very small amounts of tetrafluorethylene oligomerisation
products are obtained too.
- position under tert-butyl peroxide action with further interaction
of these radicals with tetrafluorethylene, producing oligomerisation products.
In case of using the excess of tert-butyl peroxide compounds 14 and
15 are obtained and also very small amounts of tetrafluorethylene oligomerisation
products are obtained too.
The structure of alcohols 14 and 15 is confirmed by data of 1H, 13C and 19F NMR spectra (Table. 2), IR and mass-spectrometry. In spite of the fact, that compounds 14 and 15 contain the same fragment HCF2CF2 , the structure of signals in 19F NMR spectra differs. If signals from fluorine atoms in the compound 14 have the structure of AB-system signal, then in the compound 15 it doesn't show. This is probably connected with appearance of symmetric fragment C(CH3)2OH in compound 15. Signals from fluorine atoms of 2CF2 group in this compound do not already give AB-system.
Table 2. The data of 1H, 13C and 19F NMR spectra of new partly fluorinated alcohols.
|
Compound |
NMR spectra 1H |
NMR spectra 13C |
NMR spectra 19F |
|
14 |
(H3) 6.00 (52.4; 1.2); |
115.6 (C3, 1JCF 353.5; 2JCF 25.5; |
(F2) 35.4 and 28.7 (JFF 272.1); |
|
15 |
(H3) 6.00 (52.5; 5.5); |
116.3 (C3, 1JCF 255.2; 2JCF 23.7); |
(F2) 34.1; (F3) 28.4 (52.1) |
|
16 |
(H3) 4.98 (42.5; 14); |
120.7 (C4, 1JCF 281.6; 2JCF 25.5); |
(F4) 89.5; 45.8 and 41.7 (JFF 280.3); (F2) 49.7 (JFH 41.6) |
|
17a |
(H3) 5.25 (47.0); |
121.1 (C4, 1JCF 302.1; 2JCF 25.8); |
(F4) 89.3; 41.9 and 39.0 (254.6); (F3) -49.4 |
|
17b |
(H3) 5.05 (44.0); (OH) 4.25; (H1) 4.08; (H5) 1.33 |
123.7 (C3, 1JCF 281.7; 2JCF 26.0); |
(F4) 89.2; (F2) 38.8 and 33.2 (JFF 242.9); (F3) -51.2 |
|
18 |
115.8 (C4, 1JCF 247.9; 2JCF 24.7); |
(F3) 88.3; (F4) 29.5; (F2) 27.8 |
|
|
19 |
(CHF) 5.19; (CH2) 4.61 |
145.1 (C5, 1JCF 247.6); 145.9 (C3, 1JCF 248.9); 140.2 (C1); 141 (C2,6, 2JCF 14.9); 139.6 (C4, 2JCF 13); 122 (C7, 1JCF 223.1); 121 (C11, 1JCF 281.9; 2JCF 26.2); 116 (C9, 1JCF246.6; 2JCF 25.1); 83.6 (C10, 1JCF 208.4; 2JCF 30.2); 71.7 (C8, 2JCF 36.0) |
(F7) 108.1; (F11) 89.4; (F9) 46.4 and 41.7 (JFF 281.5); (F3,5) 15.7; (F10) -49.9 (JFH 49.9) |
|
25 26 |
(CHF2) 5.93; (CH2) 4.81; (CH(CH3) 4.13; (CH3) 1.33 (O-CH2-O) 4.75; (OCH2) 3.88); (CHF) 5.03 (1JHF 44) |
115.6 (C1, 1JCF 257.8; 2JCF 6.8); 120.7 (C1, 1JCF 282.5; 2JCF 24.7); |
(F1) 36.0 and 32.2 (JFF 270.7); 25.9 and 22.9 (JFF 293.3) (F1) 89.6; (F2) 46.9 and 42.8 (JFF 291.4); -50.3 (F2) |
The position of proton signal of OH group in NMR 1H spectra depends on used solvent system - for individual liquid a proton signal of OH group of compound 14 lies at 4.31 ppm, while for 10% solution of compound 14 in CDCl3 it is situated at 3.12 ppm. The same is for compound 15: for individual liquid the proton signal of OH group lies at 3.67 ppm., and for 10% solution of compound 15 in CDCl3 the proton OH is situated at 2.69 ppm. This is because of different OH group association due to intermolecular hydrogen bonds formation.
In case of reaction of hexafluoropropylene with methyl and ethyl alcohols in the presence of tert-butyl peroxide only 2,2,3,4,4,4-hexafluorobutan-1-ol 16 and 3,3,4,5,5,5-hexafluoropentan-2ol 17 are produced respectively. The opportunity of generation of two radicals out of hexafluoropropylene by asymmetrical double bond presupposes the formation of two isomeric alcohols. In fact at interaction of hexafluoropropylene and methyl alcohol we have registered the formation of two alcohols 16 and 2-difluoromethyl-2,3,3,3-tetrafluoropropan-1-ol 18 (ratio 93:5), while the reaction with ethyl alcohol produces mixture of stable conformers 17a and 17b (ratio 58:38).
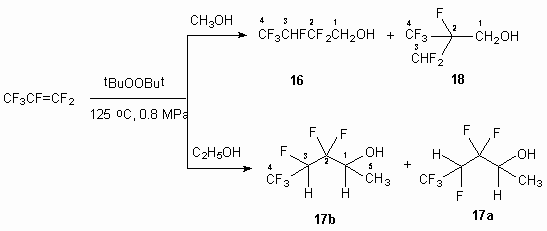
The structure of compounds 16-18 is confirmed by NMR spectroscopy data, which is interpreted taking into consideration the available information for compounds of such a kind (table 2), IR and mass-spectrometry.
Partly and completely fluorinated dialkyl ethers represent a class of compounds with interesting features, and it makes them potential fluorocontaining materials with wide field of application. Especially, their advanced thermal resistance and stability to oxidants, excellent electrophysical features of molecules in whole, increase of lubricating properties make these semi products potential for different fields of use [32-36]. Partly fluorinated ethers are used as anesthetics, special solvents, heat-transfer-dielectrics, lubricants and hydraulic liquids [36].
At the reaction of telomeric alcohols with tetrafluoroethylene and hexafluoropropylene the formation of addition products takes place by multiple bond - compounds 19 and 20 respectively.
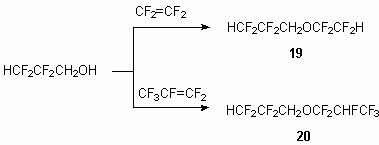
The reactions with telomeric alcohols- H(CF2CF2)nCH2OH (n = 2-4) proceed less effectively. To increase the yield of base product this process must be carried out at heating.
Telomeric alcohols are rather effective O-nucleophils, especially in the presence of bases. Thus they react with compounds, containing labile atom of haloid. It is stated, that pentafluorobenzene reacts with three moles of alcohol-telomer in the presence of KOH in DMF medium, replacing fluorine atoms in 2,4,6-positions and compounds 21 and 22 is obtained.
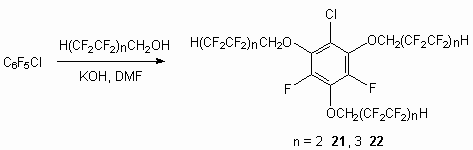
The analysis of reaction mixtures, obtained at reaction of pentafluorochlorobenzene with telomeric alcohols shows, that alkyl group doesn't really influence the nature of reaction product, and the determining factor is the conditions of process carrying out. The replacement orientation is determined by chlorine atom influence, that results in 2,4,6-trisubstituted product. Although the formation of asymmetrical products in amount of 3-5% is not excluded. However this doesn't influence the fluorine material properties, which can be effective as high-temperature heat-transfer and lubricant.
It is stated, that at interaction of equimolar amounts of 2,2,3,3-tetrafluoropropanol and freon 22 in presence of KOH dialkyl ether 23 is formed, and as by-product the compound 24 is produced.
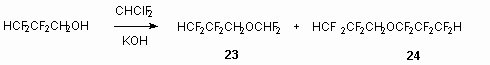
The formation of compound 24 must be proceed via intermediate generation of radical due to interaction with excess of tetrafluoroethylene.
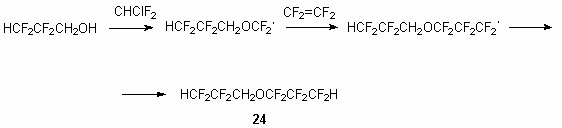
Alcohols 14-16 have all characteristics of alcohols, that is shown at example of interaction of alcohols 14 and 16 with formaldehyde in concentrated sulphuric acid, resulting in formation of ortho-ethers 25 and 26 respectively, while the reaction with alcohol 15 and formaldehyde doesn't go.
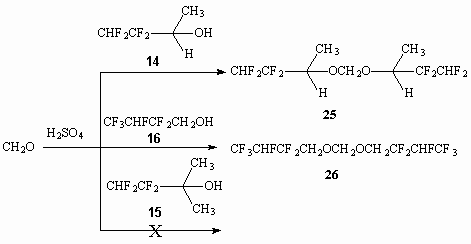
In was stated before [37], that reaction of telomeric alcohol H(CF2CF2)2CH2OH with CH2O in the presence of concentrated sulphuric acid results in formation of ortho-ether. Indeed at interaction of telomeric alcohols, obtained after reaction of tetrafluoroethylene with methyl alcohol in the presence of peroxides, with 0.5 moles of formaldehyde in concentrated sulphuric acid the respective formales 27-30 are formed.
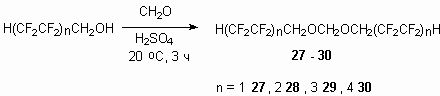
The increase of carbon chain of alcohol results in lowering of base product yield, especially it is noticeable for compound 30. To increase the yield it is recommended to carry out the process at temperatures 60-70 oC. Nevertheless the reaction has the common nature and some partly fluorinated alcohols were input there.
It should be remembered, that the action of concentrated sulphuric acid can change the direction of the process. Thus, without formaldehyde the reaction of concentrated sulphuric acid with tertiary alcohol 15 results in dehydration of last one mentioned at 50-55 oC, that results in formation of 1,1,2,2-tetrafluoro-3-methylbutene-3 31 with almost quantitative yield.
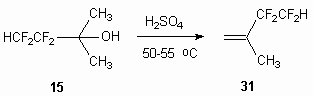
The structure of obtained products is confirmed by NMR spectroscopy, which is interpreted taking
into account the available information for compounds of such a type (table 3), and by mass-spectrometry.
In NMR 1H spectra of synthesized partly fluorinated dialkyl ethers there are the signals
of protons -CH2- and CH3 substituents, which chemical shift values are
typical for such type of hydrocarbon analogues (table 3). In NMR 19F and 13C
the signals of fluorine and carbon atoms are observed, they are typical for for hydrocarbon
skeletons, at that for fluorine atoms of CF2 groups, situated at asymmetrical carbon
atoms, the signal structures is an AB-system with JFF equal to 273-305 Hz. The main
changes happen to fluorine atoms, situated at CHF and CHF2 groups (table 3).
Table 3. The data of 1H, 13C and 19F
NMR spectra of new partly fluorinated dialkyl ethers
|
Compound |
NMR spectra 1H |
NMR spectra 13C |
NMR spectra 19F |
|
21 |
(H11) 5.13 t (50); |
39.5 (F8) 88.4; |
|
|
23 |
(H4) 6.21 t (72); |
115.2 (C1, 1JCF 264.4); |
77.5 (F1; d, 73.4); |
|
24 |
(H1) 6.22 t (72); |
115.2 (C1, 1JCF 245.0; |
77.3 (F1, d, 71.5); |
|
25 |
(H1); 5.97, (tt, 51.8: 5.0); |
115.7 (C2, 1JCF 278.9; 2JCF 30.4); |
43.9 (F6); 42.1 (F5); |
|
26 |
(H1); 5.96, (tt, 51.8; 5.0); (H10); 4.79 (s); |
115.5 (C8, 1JCF 257.7; 2JCF 30.4); |
43.8 (F8); 42.1 (F5-7); |
|
27 |
(H1) 5.88, tt 53.2; 4.8); |
115.1 (C1, 1JCF 248.7; 2JCF 27.0); |
38.4 (F2, s); |
|
28 |
(H1); 6.00, tt, 46.4; 5.4); |
115.6 (C2, 1JCF 278.9; 2JCF 30.4); |
43.9 (F3); 38.8 (F4); |
|
29 |
(H1); 5.93 (t, 54); |
115.5 (C1, 1JCF 253.1; 2JCF 26.4); |
26.0 ; 22.9 (F1, AB-system, JFF 303) |
|
30 |
(H1); 5.37 (t 51.8; 5.0); |
121.1 (C1, 1JCF 281.5; 2JCF 25.6); |
89.5 (F1, c); |
|
31 |
(H1); 5.67 (t, 50); |
135.3 (C4, 2JCF 22.5); |
46.5 (F2, c); |
For partly fluorinated dialkyl ethers, telomeric alcohols and partly fluorinated esters of carboxylic acids the researches regarding study of toxicological parameters using mice were carried out. The results are listed in table 4. According to it the studied compounds belong to third or fourth class (editor notice - Russian classification of toxicity) of moderately toxic or low-toxic compounds. It is stated, that dialkyl ethers do not have a local influence on skin and iris. The telomeric alcohols in subtoxic dose (1000 mg/kg) give the depression of respiration centers and central nervous system, showing in breaking of mice motion coordination. Given phenomenon is restored in a day.
Table 4. Some toxicological parameters of fluorine-containing compounds
|
Compound |
LD50 mg/kg |
LD100 mg/kg |
Danger class |
|
HCF2CF2CH2OH |
2320 |
3000 |
III |
|
CF3CHFCF2CH2OH |
640 |
1000 |
III |
|
HCF2CF2CF2CF2CH2OH |
1180 |
2000 |
III |
|
HCF2CF2CH2OCH2CH3 |
3420 |
6000 |
III |
|
CF3CHFCF2OCH2CF2CHF2 |
5000 |
IV |
|
|
HCF2CF2CH2OCHF2 |
5000 |
IV |
|
|
CF3CHFC(O)OCH2H5 |
5000 |
IV |
|
|
CH2[OCH2(CF2CF2)2H]2 |
5000 |
IV |
|
|
CH2[OCH(CH2)CF2CF2H]2 |
1917 |
5000 |
III |
|
HCF2CF2C(CH3)=CH2 |
1334 |
3000 |
III |
|
|
5000 |
IV |
Experimental
NMR 1H, 13C, 19F spectra were made on spectrometer Bruker WP 400 SY (400, 100, 188 MHz respectively) relatively to internal standards HMDS, C6F6 ( JCH was not measured). IR-spectra were recorded on spectrometer Specord M-80 (CCl4); mass spectra (70eV energy of ionizing electrons) were recorded on chromatograph with mass selective detector (Hewlett Packard G 1800 A GCD) (30 m column length; diameter 0.25 mm; inside coated by co-polymer - 0.25 micrometer thickness- of 5% diphenyl-95% dimethylsiloxane (HP-5); helium as a gas carrier, 1cm3/min; evaporator temperature - 280oC. The column temperature was programmed from 50 (retention time 2 min) till 280oC (retention time 5 min) at 10oC/min. All reactions were controlled using method of NMR 19F. The analysis of reaction mixtures was carried out using chromatograph LHM 72 (15 % SE-30, SKTF-803, QF-1, hromosorb W, column 4000 mm, diameter 4 mm). Compound 24 is obtained according [37].
The characteristics of new compounds and their analytical data are listed in tables 5-7, the strong
toxicity of which was determined using white mice, 22-25 g weight, using doses starting from
500 to 5000 mg/kg at oral introduction using Kerber method (table 4)
Table 5. Characteristics of partly fluorinated dialkyl ethers and analytical data.
|
Compound |
Yield, % |
BP, oC |
nD20 |
d420 |
Found,% m/z H F [M-H]+ |
Formula |
Calc, % C H F/Ì |
|
1 |
98 |
54-55 53.7 [8] |
1.3470 1.2802 [8] |
1.4200 |
26.40 2.14 62.54 181 |
C4H4F6O |
26.37 2.20 62.64 |
|
2 |
99 |
64-65 |
1.3460 |
1.3040 |
31.79 3.19 57.73 196* |
C5H6F6O |
30.61 3.06 58.16 |
|
3 |
88 |
85-86 |
1.3230 |
1.2800 |
34.16 3.90 54.41 209 |
C6H8F6O |
34.29 3.81 54.29 |
|
4 |
87 |
102-103 |
1.2930 |
1.5780 |
26.11 1.73 67.11 281 |
C6H4F10O |
25.53 1.42 67.38 |
|
7 |
60 |
95-96 95-96 [9] |
1.3200 1.3198 [8] |
1.358 |
29.78 2.46 46.86 159 |
C4H4F4O2 |
30.00 2.50 47.50 |
|
8 |
94 |
108-109 108-109 [9] |
1.3340 |
1.3040 |
34.36 3.44 44.30 173 |
C5H6F4O2 |
34.48 3.45 43.68 |
* [m/z] = [M]+
Table 6. Characteristics of partly fluorinated alcohols and analytical data.
|
Compound |
Yield,% |
BP,oC |
nD20 |
d420 |
Found,% m/z C H F M-H]+ |
Formula |
Calc, % Ì C H F |
|
14 |
94 |
111.5 |
1.3370 |
1.3716 |
33.26 4.17 51.98 145 |
C4H6F4O |
32.88 4.11 52.05 146 |
|
15 |
86 |
117 |
1.3470 |
1.3120 |
37.83 5.02 47.46 159 |
C5H8F4O |
37.50 5.00 47.50 160 |
|
16 |
79 |
114-115 |
1.3130 |
1.5710 |
25.69 2.22 62.50 181 |
C4H4F6O |
26.37 2.20 62.64 182 |
|
17a,b |
85 |
118.5 |
1.3300 |
30.24 2.98 58.40 195 |
C5H6F6O |
30.61 3.06 58.16 196 |
|
|
19 |
94 |
135-137 |
1.2910 |
1.5350 |
25.69 1.99 64.68 231 |
C5H4F8O2 |
25.86 1.72 65.52 232 |
|
25 |
87 |
89-90* |
1.3482 |
1.4000 |
35.24 4.25 50.24 303 |
C9H8F12O2 |
35.53 3.95 50.00 304 |
|
26 |
77 |
92-93* |
1.3232 |
1.6000 |
35.53 4.32 50.33 303 |
C9H12F8O2 |
35.53 3.95 50.00 304 |
* 0.5 mm Hg
Table 7. Characteristics of partly fluorinated dialkyl ethters and analytical data
|
Compound |
Yield,% |
BP,oC/mm Hg |
Found,% m/z Ñ H F [M-H]+ |
Formula |
Calc, % Ì C H F |
|
21 |
82 |
226-228/0.7 |
29.53 0.98 59.47 |
C21H9ClF26O3 |
30.07 1.07 58.95 |
|
22 |
74 |
191-193/0.4 |
27.70 0.80 63.09 |
C27H9ClF38O3 |
28.47 0.79 63.44 |
|
23 |
69 |
66-67.5 |
33.60 2.37 51.93 143 |
C4H4F6O |
33.33 2.78 52.78 144 |
|
28 |
76 |
233 |
28.29 1.75 64.64 475 |
C11H8F16O2 |
27.73 1.68 63.87 476 |
|
29 |
35 |
285 |
27.07 1.04 67.66 |
C15H9F24O2 |
26.63 1.18 67.46 |
|
30 |
39 |
191-193/0.4 |
25.46 0.99 69.47 |
C19H8F32O2 |
26.03 0.91 69.41 |
|
31 |
92 |
52-53 |
42.40 4.11 53.06 141 |
C5H6F4 |
42.25 4.23 53.52 142 |
Partly fluorinated dialkyl ethers synthesis.
1. 40 ml of absolute methyl alcohol are put into flask volume 100 ml, equipped with magnetic stirrer, thermometer, introduction tube for gaseous reagents and backflow condenser, and after that at room temperature metal sodium is added by portions of 1 g into flask, after addition gaseous hexafluoropropylene is passed through at such speed, that makes gas slippage minimal. The operation is carried out during 1.5 hour the reaction mixture is poured out into 300 ml of water, shaken and lower organic layer is separated and dried over CaCl2. The reaction mixture is analyzed using spectroscopy NMR 19F and mass-spectrometry. The mixture is distilled, and fraction with with boiling point 54-55 oC is selected (weight 33 g).
2. In a similar manner 50 ml of methyl alcohol (ethyl alcohol, isopropyl alcohol, 2,2,2,2-tetrafluoropropyl alcohol) and 5g of KOH are put into flask. Starting from 20 oC at normal pressure the flow of gaseous hexafluoropropylene is passed through, the heating of reaction mixture up to 40 oC is observed. Hexafluoropropylene is passed through till heat release stop, the reaction mixture is poured into 400 ml of cold water, all is shaken, lower organic layer is separated, washed by 100 ml of water and dried over CaCl2. Products are distilled and analyzed.
3. 10 ml of methyl alcohol and 11.2 g of solid KOH are put into rotating autoclave, the autoclave is closed and 30 g of hexafluoropropylene are condensed through valve into it. Autoclave is heated at 50 oC during 1 hour (pressure is increasing up to 4 bar), cooled, opened and the content of it is poured into water. 34.2. g (94%) of compound 1 are obtained, boiling point 54-55 oC.
Partly fluorinated dialkyl ethers hydrolysis.
1. 15 g of compound 1 (similarly compound 2) are put into flask (20 ml volume) with magnetic stirrer and during stirring 15 ml of concentrated sulphuric acid are added, the heating of reaction mixture is low. After that at stirring the reaction mixture is heated at 40 oC during 1 hour, is poured on 50 ml of ice, organic layer is separated, washed with water and dried over CaCl2. The reaction products separate by distillation.
2. Similarly, 27 g of stibium pentafluoride are put into flask and while stirring 15 g of compound 2 are added by portions. The temperature should not exceed ambient. The mixture at room temperature was stirred during 1 hour, poured on 50 ml of ice. Organic layer was separated, washed with cold water and dried over CaCl2. Product was distilled and fraction with BP 95-96 oC was selected (weight 8 g).
Action of potassium hydroxide on partly fluorinated dialkyl ethers.
40 g of compound 1 (analogously to this compound 2), 50 ml of dry dioxane are put into flask (volume 100 ml), equipped with magnetic stirrer, thermometer and backflow condenser, and while stirring at room temperature milled KOH is powdered by portions, the mixture heating is observed. The reaction mixture is being heated at 40 oC at stirring during 2 hours and products of reaction are being distilled out from the flask. The yield of compound 5 is 82 %, boiling point. 51-52 oC, nD20 1.3208, d420 1.3601 (according to [9] boiling point. 51.5 oC/743, nD20 1.2970, d420 1.3595) and yield of compound 6 is 78 %, boiling point 71-72 oC, nD20 1.3210, d420 1.2930 (according to [8] boiling point 71-72 oC/743, nD20 1.3103, d420 1.2884).
General Method of Partly Fluorinated Alcohols Synthesis
Ethyl alcohol (or iso-ppropyl alcohol) and di-tert-butylperoxide in proportion 3-15,4% from mass of alcohol are charged into reactor. Reactor is being heated up to 150 oC in the presence of polyfluorinated alcohol taking in quantity 0,2-1,2% from mass of alcohol and the introduction of tetrafluoroethylene (hexafluoropropylene) at the pressure of 9-15 bar is started. Mole ratio fluoroolefine-initiator is 7.2-28.2:1 and fluoroolefine-alcohol - 1:15. The reaction time is 4 hours, after that excess of olefin is discharged and reaction mixture is distilled. A first, distill the excess of ethanol, after that CaCO3 is added to reaction mixture and alcohol 14 with purity 99,9% is distilled out (analogously alcohol 15). Alcohols 16-18 was produced from methyl and ethyl alcohols at the same conditions.
The Interaction of 2,2,3,3-tetrafluoropropyl alcohol and freon 22.
The reaction was carried out in stainless steel reactor (volume 2 dm3), equipped with stirrer, heating, temperature and pressure control, and freon-22 supply systems. 150 g KOH are dissolved in 800 g of 2,2,3,3-tetrafluoropropyl alcohol and put into reactor, which was vacuumized further till residual pressure of 1 bar, and then the heating and stirrer of reactor are turned on, freon 22 is fed into reaction volume till the pressure reaches 1-1.5 bar and the mixture is heated slowly till the temperature reaches 50 oC, at which the reaction with heat evolution starts. In the process of synthesis the pressure in the reactor is kept with the range of 8-12 bar, and the temperature is kept within 60-70 oC, for this purpose the reactor is cooled from time to time. The main criteria of reaction progress evaluation is the pressure drop in reactor when freon 22 supply valve is closed. The moment of reaction finish is predetermined by stop of pressure drop or even by its rising. After end of synthesis the reactor is cooled, residual pressure (2-4 bar) is blown away and after discharge the raw material of 1000-1600 g is obtained, with main compound content of 40-50 % vol. The obtained raw material is washed through with distilled water and then is exposed to fractional distillation on the column, filled with nichrome curls or ceramic rings. The length of the column is 1 m. After distillation the fraction, containing 98% of base product (compound 23), is obtained, at that the selection of fraction is carried out at column top temperature of 65-70 oC, and compound 24 is obtained in small amount.
The Interaction of Pentafluorochlorobenzene and Telomeric Alcohols.
The mixture: 41 g of pentafluorochlorobenzene, 199 g of telomeric alcohol H(CF2CF2)3CH2OH [or H(CF2CF2)2CH2OH ] , 40 g of KOH and 150 ml of dimethylformamide was mixed at 95 oC during 20 hours and was left till morning. The reaction mixture was poured out into the water, the organic layer was separated, washed through with water, and dried over MgSO4. Main products 22 (or 21) were obtained using distillation.
The Reaction of 2,2,3,3-tetrafluoropropyl alcohol with hexafluoropropylene
The reaction is carried out in reactor, described above, under analogous conditions. 800 g of 2,2,3,3- tetrafluoropropyl alcohol, 150 g of KOH and 50 g of triethylamine are put inside. As the reaction runs roughly with calorification, it is necessary to keep the temperature inside the reactor within 40-50 oC using constant cooling, and the pressure is impossible to rise higher than 4 bar even at hexafluoropropylene feeding valve opened all the time. Under these conditions the synthesis time is 15-30 min., that is 2-3 times shorter than synthesis time with freon 22. The mass of raw material obtained is 1500-2000 g, and main compound 20 content in it is 40-50 % vol. The extraction of base product 20 is carried out the same way as described above: boiling point 102-103 oC ( according to [38] boiling point. 102-103; nD20 1.293; d420 1.578).
General Method of Formale Synthesis on the Basis of Telomeric Alcohols and Formaldehyde
200 ml of concentrated sulphuric acid are poured into three-necked flask, of 0.5 dm3 volume, equipped with stirrer, the stirring mode is turned on, 15 g of paraform are also poured into it and 132 g of 2,2,3,3-tetrafluoropropyl alcohol (analogously to another telomeric alcohol) are added. The flask is put into cold aqua bath to prevent heating and to control temperature at 20 oC, it is kept at constant stirring for 3 hours, poured out on ice, the organic layer is separated, dried over CaCl2 and fractioned (it is better to distill at lowered pressure). The content of main compound in base fraction is 93-95 % (vol.) at that. The compounds 27-30 are obtained. Compounds 25 and 26 are obtained the same way.
The Dehydratation of 2,2,3,3-tetrafluoro-1-methylbutan-2-ol
All syntheses were carried out using facility, consisting of three-necked flask volume 0.5 dm3, equipped with backflow condenser, the base product selection system in the process of synthesis. 170 ml of concentrated sulphuric acid and 50 g of 2,2,3,3-tetrafluor-1-methylbutan-2-ol 15 were put into reactor and heated up to 50-55 oC and then the base product 31 selection was started into cooled collector. The period of synthesis is 20-30 min. As a result 25-30 g of 1,1,2,2-tetrafluoro-3-methylbutene-3 (31) with purity 99.8-99.9% (vol.) are obtained. The additional purification is not required.
Conclusions
- The opportunities of partly fluorinated alcohols synthesis on the basis of tetrafluoroethylene and hexafluoropropylene reactions with alcohols in the presence of peroxides are extended.
- Partly fluorinated alcohols in the presence of bases show nucleophilic properties regarding compounds with high electrophilicity, that allows to work out on their basis convenient and simple obtaining methods of partly fluorinated dialkyl ethers and other derivatives.
- The reaction of telomeric alcohols with formaldehyde in the media of concentrated sulphuric
acid produces formales, which may be used as high temperature heat-transfers, and their
further additional fluorination results in fully fluorinated dialkyl ethers which are
of interest as effective dielectrics with wide interval of boiling points.
References
[1] Orlov A.P., Schavelev V.B., Korolkov D.N. // The 3d International Conference “Chemistry, Technology and Application of Fluorocompounds”, June 6-9, 2001. St. Petersburg, Russia. Abstracts. P1-34. P. 56.
[2] Orlov A.P., Schavelev V.B., Barabanov V.G., Korolkov D.N. // The 3d International Conference “Chemistry, Technology and Application of Fluorocompounds”, June 6-9, 2001. St. Petersburg, Russia. Abstracts. P1-36. P. 58.
[3] Lemaire B., Surbled M., Mengal P.P., Monpon B.M. // Appl. 2771408 France (1999)
[4] Hanada T., Tsuzaki M. / Appl. 2358189 UK (2001)
[5] Falcone S.J. // Pat. 6187954 US (2001).
[6] Marchionni D., Guarda P.A. / Pat. appl 97102618/04 RU
[7] Knunyants I.L. , Schekotichin A.I., Fokin A.V. // Izv. Akad. Nauk SSSR. Otd. Khim. N. 1953. № 2. P. 282-289.
[8] Rendall J.L., Pearlson W.H. // Pat. 2862024 US (1958)
[9] Bagramova M.D., Cheburkov Yu.A., Dyatkin B.L., Petrovskii P.V., Knunyants I.L. // Izv. Akad. Nauk SSSR. Ser. Khim. 1967. № 3. P. 611-614.
[10] Dyatkin B.L., Konstantinov Yu.S., Lanzeva L.T., Bekker P.A., Knunyants I.L. // Zh. Org. Khim. 1967. V. 3. N 6. P. 1006-1011
[11] England D.C., Solomon L., Krespan C.G. // J. Fluorine Chem. 1973. V. 3. N 1. P. 63-89
[12] Popova L.M., Volod`kov V.N., Prytkova O.A., Drozdov V.V., Cvetkov O.M., Riabinin N.A. // The 2nd International Conference “Chemistry, Technology and Applications of Fluorocompounds”. Sept. 23-26, 1997. St. Petersburg, Russia. Abstracts. Z3-9. P. 123.
[13] Hansen J.C. // Pat. 5474657 US (1995)
[14] Jackson S.C., Resnick P.R., Swearingen S.H. // Pat. 5516946 US (1996)
[15] Novikova M., Zakharov V., Denisov A., Jukova V. // J. Fluorine Chem. 1992. V. 58. N 2-3. P. 169.
[16] Gubanov V.A., Tumanova A.V., Dolgonolsky I.M. // Zh. Obshch. Khim. 1965. V. 35. N 2. P. 398-400.
[17] Yamaguchi F., Takaki S., Yoshizawa T., Ogura E., Katsube T. / Pat. 6187969 US (2001)
[18] Uklonskii I.P., Denisenkov V.F., Il`in A.N., Ivanova I.M., Bakhmutov Yu.L., Mineev S.N. // Pat. 2150459 RU (2000); Chem. Abstr. 2002. Vol. 136. 218614b.
[19] Blickle P., Heumueller R., Hintzer K., Schwertfeger W., von Werner K. / Ger.Offen DE 3915759 (1990); Chem. Abstr. 1991. Vol. 114. 184789r.
[20] Rahimov A.I., Vostrikova O.A., Ermakov A.V. / Tez. dokl 5 mezhdunarodnoj konf. «Naukoemkiekhimicheskie tekhnologii», 19-21 maya 1998g., Yaroslavl, t. 1, s. 141-142
[21] SkibidaI.P.,SakharovA.M.,BakhmutovJ.L.,DenisenkovV.F.,MartynovaN.P. /Pat. 5495034US (1996)
[22] IchiharaK.,Aoyama H. / Patent 1085006 USA (2001)
[23] Nguyen T., Wakselman C. // Synth. Commun. 1990. V. 20. N 1. P. 97-99.
[24] Maksimov B.N., Kosareva L.N., Ryabinin N.A. // Pat. 2107751 RU (1993);
[25] Szlavik Z., Tarkanyi G., Skribanek Z., Vass E., Rabai J. // Org. Lett. 2001. V. 3. N 15. P. 2365-2366; Chem. Abstr. 2001. Vol. 135. 210736b.
[26] A.I. Rahimov. Khimiya i tekhnologiya ftoroorganicheskih soedinenij. M.: Mir. 1986, 272 s.
[27] Wada A., Tanaka H., Tanabe K., Yamagishi N., Toma T. / Appl. 1191009 EP (2002);
[28] Yashizawa T., Takai S., Yasuhara T., Yokoyama Y. / Appl. 1179521 EP (2002); Eur. Pat. Appli. EP 967193 (1999); Chem. Abstr. 2000. Vol. 132. 51457f.
[29] Oharu K. / PCT Int. Appl. WO 02 28807 (2002); Chem. Abstr. 2002. Vol. 136. 294534r.
[30] Ishihara K., Homoto Y., Baba N. / Jpn Kokai Tokkyo Koho JP 53505 (2002); Chem. Abstr. 2002. Vol. 136. 167088y.
[31] Okamoto H. / PCT Int. Appl. WO 02 16295 (2002); Chem. Abstr. 2002. Vol. 136. 199936q.
[32] Synthesis Fluorine Chemistry. / Eds. G.A. Olah, R.D. Chambers, G.K.S. Prakash. New York : John Wilei & Sons Inc. 1992. P. 143-164.
[33] Lagow R.J., Nagase S. // Preparation, Properties and Industrial Application of Organofluorine Compounds. / Ed. R.E. Banks. Chichester. 1982. P. 19-44.
[34] Lagow R.J., Lin T.-Y., Roesky H.W. et al. // Inorganic Fluorine Chemistry. / Eds. J.S. Thrasher, S.H. Strauss. ACS Symposium Series 555. Washington, DC : Am. Chem. Soc. 1994. P. 216-236.
[35] Sung K.-G., Lagow R.J. // J. Am. Chem. Soc., 1995. V. 117. N 15. P. 4276-4278.
[36] Brodbelt J., Maleknia S., Liou C.-C., Lagow R.J. // J. Am. Chem. Soc. 1991. V. 113. N 15. P. 5913-5914.
[37] Hill M.E., Shipp K.G. / Pat. 3526667 US (1970);
[38] Fokin A.V., Komarov V.A., Kolomiets A.F., Rapkin A.I., Verenikin O.V., Potatina T.M. // Izv.Akad. Mauk SSSR. Ser. Khim., 1977. N 9. P. 2141-2146.
This work was carried out under innovation projects financial support by Siberian
branch of Russian Academy of Science (grant N37, 2003)
Fluorine Notes, 2003, 30, 3-4