Fluorine Notes, 2003, 28, 1-2
Perfluoroalkanesulfonic acids derivatives:
synthesis and application
G.G. Furin
Novosibirsky Organic Chemistry Institute of N.N.Vorozhtsov , Siberian department of Russian Academy of sciences,
Ak. Lavrentiev avenue, 9, Novosibirsk, Russia, 630090
Fax: +7 3832 344752
E-mail : furin@nioch.nsc.ru
Abstracts
Information of the last 10 years regarding methods of synthesis and properties of perfluoroalkanesulfonic acids and some of its derivatives is listed and analyzed here. The opportunities of using the perfluoroalkane halides and syntons on the basis of perfluoroalkylsilicon derivatives for these purposes are uncovered. The main attention is paid to practical aspects of perfluoroalkanesulfonic acids salts and bis(perfluoroalkylsulfonyl)imides using as catalysts of different processes, electrolytes, ionic liquids and N-F bond containing compounds as mild fluorinating reagents. The ways of using of perfluoroalkanesulfonic acids derivatives in organic synthesis are discussed.
Table of contents
1.Introduction. The role of organic compounds in the synthesis of semi-products and the creation of new materials on their basis.
2. Perfluoroalkanesulfonic acids synthesis
2.1. Electrochemical fluorination
of alkyl derivatives of hexavalent sulfur
2.2. The use of perfluoralkyl
iodides in the synthesis of perfluoroalkanesulfonic acids
2.3. The reactions of fluorocontaining C-nucleophilic reagents with haloid derivatives of sulfur
2.4. The use of trimethyl(trifluoromethyl)silane in the synthesis of trifluoromethylsulfonic acid and its derivatives.
3. The synthesis and characteristics of perfluoroalkanesulfonic acids derivatives
3.1. Haloanhydrides of perfluoroalkanesulfonic acids
3.2. Bis(perfluoroalkylsulfonyl)imides and their use in fluoroorganic synthesis
3.3. Perfluoroalkanesulfonic acids salts as catalysts of several chemical processes.
3.4. The synthesis of N-F containing compounds.
4. The new applications of perfluoroalkanesulfonic acids derivatives
4.1.
Electrolytes and ionic liquids on the basis of bis(perfluoroalkylsulfonyl)imides
4.2. Membranes on the basis of perfluoroalkanesulfonic acids
Conclusion
References
Derivatives of quadrivalent and hexavalent sulphur, containing haloid atoms, exchange halogen groups for different functional ones when nucleophilic reagents are acting. As a result of this the main task is the generation of fluorine-contraining carbanions and their introduction into reactions with sulphur compounds. One of the approaches which are based on one of available and produced compounds is generation of carbanions out of perfluorolefines under the action of alkali metals fluorides. Thus sulfuryl fluoride joins to fluoroolefines in presence of alkali metals fluorides in polar aprotic solvent, producing respective sulfofluorides or sulfones [59].

The mechanism of these changes is in initial addition of fluoride-ion with fluoroolefine and generation of carbanion, reacting with sulfuryl fluoride.
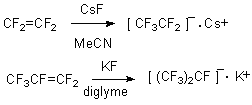
Similarly, perfluoro-isopropylsulfonyl fluoride with 72% yield was obtained from hexafluoropropylene and sulfuryl fluoride at the presence of KF [60].
2.4. The application of trimethyl(trifluoromethyl)silane in the synthesis
of trifluoromethane-sulfo-acid and its derivatives.
The other method is using of synthones on the base of IV group elements. Thus if interaction of trimethyl(trifluoromethyl)silane with SO2 during absence of anionic initiators doesn't take place then in presence of fluoride-ion sources (for example, tetrabutylammonium fluoride (TBAF)), the formation of tetra(n-butyl)ammonium trifluoromethanesulphite [61] takes place. It was found that Me3SiONa in tetrahydrofurane was able to employ instead TBAF. The further oxidization of this salt by 30% hydrogen peroxide results in formation of trifluoromethanesulfonate or free trifluoromethanesulfo-acid. Me3SiONa is rather effective as initiator of this reaction. Though total yield of trifluoromethanesulfo-acid is not high (up to 30 %). At the same time this method can be competitive regarding electrochemical fluorination method.
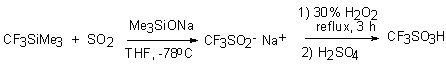
In the work [62] it was shown that reaction CF3SiMe3 with 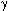 -SO3 in freon 113 runs rather easy at temperature gradient from –196 to 25°C with
formation of trimethylsilyl ester of trifluoromethanesulfo-acid.
-SO3 in freon 113 runs rather easy at temperature gradient from –196 to 25°C with
formation of trimethylsilyl ester of trifluoromethanesulfo-acid.
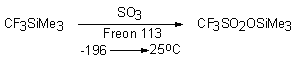
It's
natural that CF3SiMe3 at conditions of nucleophilic catalysis by fluoride-ion
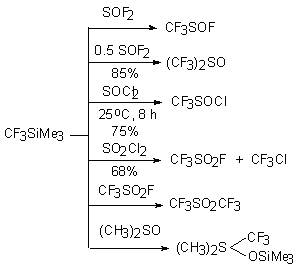
react with sulfo-compounds containing labile atom of halogen. Thus during reaction with SOF2 (mole ratio of reagents 1:1 and 1:2) CF3SOF and (CF3)2SO
was produced. Similarly this system reacts with SOCl and SO2Cl2 [63].
Analogously to this method trifluoroetylenesulfo-acid can be obtained [64].
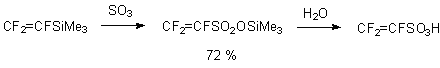
3. The synthesis and properties of perfluoroalkanesulfo-acids derivatives.
3.1. Haloidanhydrides of perfluoroalkanesulfo-acids
The main derivatives of perfluoroalkenesulfo-acids are its haloidanhydrides. Their chemistry
is rather representative. That's why we give only such examples, that result in formation
of strong acids and complexes.
Most reactive derivatives of perfluoroalkenesulfo-acids
are their chloroanhydrides. The efforts for creating simple and convenient method of
transforming corresponding perfluoroalkenesulfo-acids into their chloroanhydrides were
undertaken. The most convenient method is interaction of for example trifluoromethanesulfo-acid
with mixture of PCl3 and Cl2 (1:1) or PCl3, POCl3,
Cl2 (1:1,9:1,1) at 81 oC in 4 hours. The yield of CF3SO2Cl
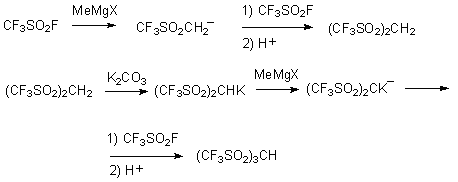
was 89,7 % [65]. Trifluoromethanesulfonyl fluoride was used for synthesis of C-H acids
: (CF3SO2)2CH2 [66] (the review of synthesis
methods is listed in the work [67]), (CF3SO2)3CH [68]
:
Tris(trifluoromethanesulfonyl)methane - is a strong acid in gaseous phase [69].
During
action of methyllithium perfluoroalkylsulfonylchloride in diethyl ether the lithium salt
of tris(trifluoromethanesulfonyl)methane is formed with the yield 31 % while interaction
with anhydrous ammonia in isopropyl ether produces trifluoromethanesulfonamide [70].
During interaction of perfluoroalkanesulfonyl fluorides with dimethylamine or halogensulfonamides
the fluorinated sulfonamides X(CYZ)mSO2N(CRR1R2)2 ( X = H, F, Cl; CnF2n+1; m = 1-9) with nearly quantitative yield
are produced [71]. These compounds are used as difficult-to flame dissolvents in electrolytes
of electrochemical elements, consisting of cathode, anode, separator and electrolyte.
Particularly lithium batteries and super condensers are used as such elements.
Since the bond sulfur-chlorine in trifluoromethanesulfochloride is easily is exposed to nucleophilic
agents action compare to sulfur-fluorine bond the main chemistry of this class is developed
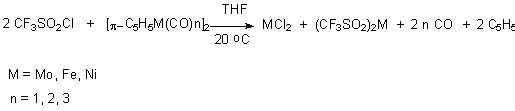
on perfluoroalkenesulfochlorides. It is shown [72-73], that trifluoromethanesulfochloride
reacts with bis( -cyclopentadienyl)metal
carbonyles in polar medium like tetrahydrofurane and metal complexes are formed .
-cyclopentadienyl)metal
carbonyles in polar medium like tetrahydrofurane and metal complexes are formed .
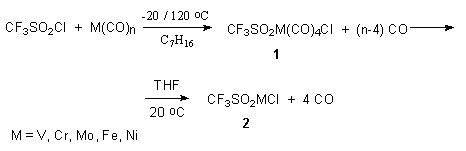
At the same time the interaction of perfluoroalkenesulfochlorides with carbonyls of V-, VI [74], VIII [74] groups metals results in formation via unstable compound 1 of sulfinate-O,O'-metalchlorides 2.
Direct hydroxylation of olefine, obtained by reaction of perfluoroiodide and allyl-acetate, with further hydrolysis by HSi(OEt)3 action in presence of palladium catalyst produces sulfofluoride.
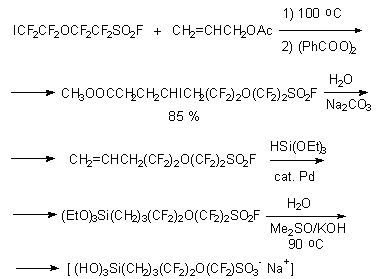
The last one at hydrolysis allows to obtain new effective reagent (perfluorosulfonate/trisilanol) which effective as catalyst of alkenes isomerisation, alkylation and acylation reactions etc [75].
![Reaction Product yield, % cat. Nafion resinNR 50 Amberlyst 15 Alkylation of toluene by heptene (100oC, 2 hours) 99 5 13 Butene-1 isomerization (50oC) 95 14 47 Acylation of m-xylene (140oC, 6 hours) 89 90 - Alkylation of benzene by dodecene-1 (80oC, 1 hour) 43 3 5 Constant ([H+]-1hours-1) of -methylstyrene dimerization (50oC) 1000 0,1 0,6](/public/images/28_1/t1.png)
Fluorine Notes, 2003, 28, 1-2
