Fluorine Notes, 2003, 28, 3-4
New approaches to nitrogen fluoride synthesis
Message 3
Offer regarding setting of nitrogen fluoride unified production
V.M. Andrushin
Along with the most extensively studied reaction of nitrogen trifluoride with fluorine acceptor:
2NF3 + 2M ->N2F4 + 2MF,
where hydrogen, arsenic, carbon, antimony, nitric oxide and other compounds of different classes;
the group of methods, based on oxidization of difluoroamines, the source for which is N,N
difluoro -derivatives of urea, carbamate, mono- or difluoro-derivatives of sulphuric acid, which are obtained by direct fluorination of aqueous solutions of corresponding amides using
diluted fluorine at low temperatures, is of practical interest for tetrafluorohydrazine
synthesis.
For oxidization of difluoroamines it is offered to use iodine, bromine, aqueous solutions of chromium, iron, cerium, potassium permanganate salts and others. The extraction of
difluoroamines out of solutions is necessary to obtain best results regarding yield and fineness of
tetrafluorohydrazine in these methods.
This is a serious obstruction for their manufacturing application, because difluoroamines is explosive, especially at changes of phase and thats why they are mainly used for preparative purposes.
The methods, provided for difluoroamines oxidization in the moment of difluoroderivatives extraction directly from N, N solutions are thought to be more perspective.
In literature the oxidization of N,N difluorcarbamate by chrome oxide at 50Ñ and N, N difluor-urea by aqueous solutions of such compounds as K2CrO4, KMnO4, CrF3, Fe(NO3)3, Mn2(SO4)3, KFe(SO4)2, FeNH4(C2O4)2, FeNH4(SO4)2, others is described but here there is a number of technological and ecological difficulties, low reproducibility of results.
In the view of technological requirements and economical expediency the most effective scheme for the arrangement of tetrafluorohydrazine production is the following:
NH2CONH2 -> NF2CONH2 -> HNF2 + CO2 -> N2F4 + CO2 -> N2F4
The method is based on a cheap, available raw materials, it is not complicated in hardware implementation. The main difficulty of practical implementation of this scheme is the formation of
equimolar mixture of tetrafluorohydrazine and carbon dioxide, which separation using physical methods is hardy realized because of closeness of
tetrafluorohydrazine boiling point and CO2 sublimation point
(-74,2oÑ è 78,5oÑ, respectively ) We have studied the opportunity of carbon dioxide elimination in
tetrafluorohydrazine using traditional chemical method coupling the carbon dioxide with aqueous alkali solution.
It is known, that tetrafluorohydrazine , being a strong oxidant energetically reacts with many of organic and non-organic compounds. It is most rational to carry out the dioxide elimination in
tetrafluorohydrazine by treatment of
N2F4 + CO2
mixture using aqueous alkali solutions at medium temperature and brief contact. The search of such conditions was the aim of the present work. The reactions of carbon dioxide and
tetrafluorohydrazine with aqueous solutions of potassium hydrate are studied in a wide range of conditions: temperature range 0
800Ñ , alkali concentration range 5 50%, time of contact from 0,5 to 20 seconds in reactors made of glass and stainless steel. The reactor was cylinder, filled with
filling and was equipped with casing and fricative bubbler.
It was determined, that the degree of tetrafluorohydrazine decay essentially increases when temperature and alkali concentration increases. For the purposes of illustration picture 1 represents the results of
tetrafluorohydrazine interaction with potassium hydrate aqueous solutions at the contact time of 1,2 seconds.
Tetrafluorohydrazine is rather resistant to alkali aqueous solutions at low temperatures in studied conditions. Thus at temperature up to
250Ñ in 5-30% potassium hydrate aqueous solutions the tetrafluorohydrazine
decay doesnt exceed 0,1%. In concentrated alkali (48%) the interaction takes place as early as at ïðè
00Ñ (0,4%), and at 800Ñ the tetrafluorohydrazine decay reaches 19,5%.
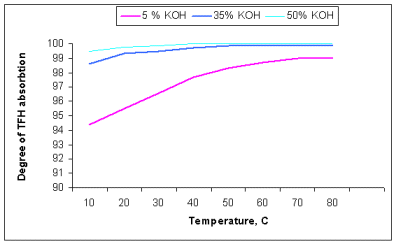
The degree of carbon dioxide absorption was determined at the same conditions. The results are presented at the picture 2. As you can see from data obtained the full absorption of carbon dioxide in 50% solution is reached as early as at
100Ñ, in 30% solution at about 35-400Ñ, and in 5% solution even at
800Ñ the degree of absorption is 99,4%.
Our apprehensions concerning the fact, that during the treatment of tetrafluorohydrazine mixtures with carbon dioxide using aqueous alkali the degree of decay may increase in some way because of local overheating didnt prove to be true. The obtained analysis results allow us to recommend using of potassium hydrate aqueous solutions of 25-35% concentration at 10-25 oC and short term reagents contact for tetrafluorohydrazine. The developed scheme of carbon dioxide elimination in tetrafluorohydrazine essentially simplifies the process of tetrafluorohydrazine obtaining from difluoromines and makes it attractive for detailed technological developmental work and implementation. It was found out before hand that the presence of carbon dioxide doesnt impede the difluoromines oxidization process.
Thus the total scheme of tetrafluorohydrazine obtaining includes the following main steps:
- fluorination of urea's agues solution by diluted fluorine a 0-50oC
- acids decomposition of N'N-fluorourea by sulfuric aid at heating
- oxidation of difluoroamine and carbon dioxide mixture by agues solution of FeCl3 without prior purification of difluoroamine
- purification of difluoroamine from CO2 by KOH
- drying and packaging of tetrafluorohydrazine
The developed scheme of carbon dioxide elimination in tetrafluorohydrazine essentially simplifies the process of tetrafluorohydrazine obtaining from difluoroaminesand makes it attractive for detailed technological developmental work and implementation.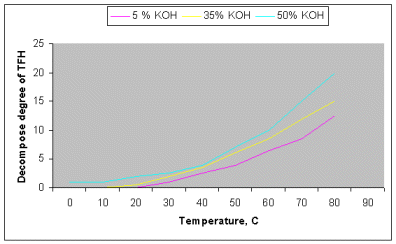
Pic.1 .
Pic.2.
Fluorine Notes, 2003, 28, 3-4
