Fluorine Notes, 2003, 27, 1-2
Perfluoroalkanesulfonic acids derivatives:
synthesis and application
G.G. Furin
Novosibirsky Organic Chemistry Institute of N.N.Vorozhtsov , Siberian department of Russian Academy of sciences,
Ak. Lavrentiev avenue, 9, Novosibirsk, Russia, 630090
Fax: +7 3832 344752
E-mail : furin@nioch.nsc.ru
Abstracts
Information of the last 10 years regarding methods of synthesis and properties of perfluoroalkanesulfonic acids and some of its derivatives is listed and analyzed here. The opportunities of using the perfluoroalkane halides and syntons on the basis of perfluoroalkylsilicon derivatives for these purposes are uncovered. The main attention is paid to practical aspects of perfluoroalkanesulfonic acids salts and bis(perfluoroalkylsulfonyl)imides using as catalysts of different processes, electrolytes, ionic liquids and N-F bond containing compounds as mild fluorinating reagents. The ways of using of perfluoroalkanesulfonic acids derivatives in organic synthesis are discussed.
Table of contents
1. Introduction. The role of organic compounds in the synthesis of semi-products and the creation of new materials on their basis.
2. Perfluoroalkanesulfonic acids synthesis
2.1. Electrochemical fluorination of alkyl derivatives of hexavalent sulfur
2.2. The use of perfluoralkyl iodides in the synthesis of perfluoroalkanesulfonic acids
2.3. The reactions of fluorocontaining С-nucleophilic reagents with haloid derivatives
of sulfur
2.4. The use of trimethyl(trifluoromethyl)silane in the
synthesis of trifluoromethylsulfonic acid and its derivatives.
3. The synthesis and characteristics of perfluoroalkanesulfonic acids derivatives
3.1. Haloanhydrides of perfluoroalkanesulfonic acids
3.2.
Bis(perfluoroalkylsulfonyl)imides and their use in fluoroorganic synthesis
3.3. Perfluoroalkanesulfonic acids salts as catalysts of several chemical processes.
3.4. The synthesis of N-F containing compounds.
4. The new applications of perfluoroalkanesulfonic acids derivatives
4.1. Electrolytes and ionic liquids on the basis of bis(perfluoroalkylsulfonyl)imides
4.2. Membranes on the basis of perfluoroalkanesulfonic acids
Conclusion
References
1. Introduction. The role of organic compounds in the synthesis of semi-products and the creation of new materials on their basis
During last decades the organofluorine compounds chemistry had gained a rather high rating due to its wide range of practical use. For example we'll point out the problems of ozone-safe compounds and artificial blood substitutes. The modern conception of researches reaching the level of international cooperation is being formed. Today it is a question of working out the uniform approaches to studying of fundamental bases of organic framework perfluorination influence on its physical and chemical characteristics.
It's natural because at every stage of technical progress the role and direction of fundamental researches to a great extent is initiated by society’s needs in new materials, which are impossible without creation of fundamentally new materials with advanced consumer characteristics, which are able to work in much more severe conditions. In this regard fluorine-containing materials have an exceptionally important role, they can't be found in nature but they are able to bring into molecules new fundamentally different from other elements characteristics. However the understanding of functional changes in the structure of organic molecule at introduction the fluorine atoms into it is not sensible and deep enough. How do the fluorine atoms influence the characteristics of molecule in whole? How do such compounds function in the organism of animal and man? How do they influence the vital functions of plants? Nevertheless the scientific approaches allowing to evaluate these questions and give a definite explanation has been already created. The production of fluorocontaining materials depends not only on the level of our knowledge but also on perspective of practical using, which is determined by the development of technical progress. The correct placing of prioties, the choice of key base compounds as a basis for production of materials, the perfection of technological decisions determines to great extent the tasks of production. Their solution allows to satisfy the needs of society on the base of basic materials fast.
The development of methods of obtaining of organic compounds with certain structure and specific characteristics is one of the central problems of organic chemistry. This is particularly important conformably to new classes of organic compounds, where nowadays the information regarding their characteristics which is available may be limited or inessential. Because of this the organic compounds, containing fluorine atoms, require rapt attention and conduction of wide researches owing to their specific influence that fluorine atoms have on characteristics of these compounds [1,2]. For the past years the understanding of fluorine compounds characteristics uniqueness has increased and many new tendencies of using appeared. The introduction of fluorine atoms into organic compounds is of big scientific and practical interest when creating new high-performance biologically active compounds and materials, possessing unique properties [3]. It is stated that these compounds posses many important aspects for practical application. The conduction of these researches has noticeably improved synthetic fluorine chemistry: plenty of new reagents and methods of synthesis were developed.
In the view of practice it is very important for synthesis methods of necessary fluorocontaining compounds with certain functional fragments to be noted for the simplicity of carrying out the process regarding reagents as well as conducting technique, for the good reproducibility, yields high enough and regio-selectivity. This is a guarantee of finding a way for study of chemical characteristics of new synthesized compounds as well as discovering of their application perspectives. In particular new materials, possessing the complex of unusual characteristics, enhancing the organic system itself are the most important task for synthetics chemists.
The author realizing that to cover all the tendencies of synthesis of fluorocontaining hexavalent sulfur
derivatives is impossible concentrated his attention on systems, containing perfluoroalkylsulfonyl
groups as substitutes.
Perfluoroalkanesulfonic acids are widely used in organic synthesis and
their salts as intermediates in surface active compounds synthesis. Perfluorinated derivatives of
alkanesulfonic acids are able to form stable foam even in the presence of strong oxidant like concentrated
chrome and sulphuric acid [4]. Due to this characteristic they are efficiently used as additives
for electrolytes at electrochemical refining of nickel and chrome. Surfactants on the basis of perfluoroalkanesulfonic
acids are very efficient agents, lowering the surface tension (to 15 din/cm). Low concentrations
necessary for such efficiency of these surfactant give big advantages in respect to environment.
These compounds decrease the surface energy of plastics and add characteristics for unfriction, inadhesiveness
and antisoiling properties to the compounds. Such solutions are produced in commercial quantities
and have found an application for practice. Perfluorinated bi-functional organic acids are rather
strong ones and can be potentially used for creating of ingredients of electrolytes of fuel elements,
particularly lithium batteries and accumulators.
The organization of surface active materials production on base of perfluoroalkanesulfonic acids will allow to obtain the characteristics noticeably higher than such ones for perfluorinated carboxylic acids which field of application is now huge and growing all the time. These surface active materials will find their application as new extracting materials and metal-dressing for non-ferrous metallurgy, for creation of fire-fighting compositions, they also can be used during electrofining of metals, creation of effective detergents, high-temperature lubricants, for natural materials treatment particularly cotton and cellulose for the purpose of their incombustibility, in the technology of semiconductor devices for the purpose of lowering the surface tension of liquids.
Perfluoroalkanesulfonic acid is one of the strongest acid and it is successfully used as catalyst of different chemical processes, at that in a number of cases and new reactions. For example, as catalyst for reactions of allyltributyltin with carbonyl compounds, giving homoallyl alcohols [5].
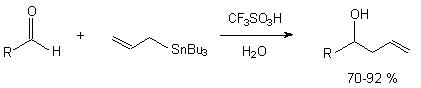
Trifluoromethanesulfonic acid is resistant to fluorine action, that is used for conducting of the direct fluorination process of aromatic compounds [6].
2. Perfluoroalkanesulfonic acids syntesis.
2.1. Electrochemical fluorination of hexavalent sulfur derivatives.
Electrochemical fluorination method of sulfur organic derivatives was the main one, used for obtaining of perfluorinated alkanesulfonic acids syntesis [3]. As a rule in this case the source material is sulfur hydrocarbon derivatives. Among important practical applications of electrochemical fluorination method there is working-off of obtaining method of perfluoroalkanesulfofluorides and perfluoroalkanesulfonic acids, starting from derivatives of alkanesulfonic acids, because the derivatives of perfluoroalkanesulfonic acids have special interest for their industrial applications. This approach became the main method of perfluoroalkanesulfonic acids synthesis in the present time [7-11]. Perfluoroalkanesulfonic acids fluoroanhydrides formed during this are hydrolyzed by alkali up to alkaline salts of perfluoroalkanesulfonic acids, which can be used directly as surface active materials. Electrolysis is held using nickel electrodes in anhydrous hydrogen fluoride at voltage below the one of gas fluorine evolving (4,5-6,0 V) and current density about 0,02 A/m2 .
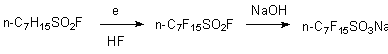
Reviews on electrochemical fluorination of sulfur containing compounds, including the ones containing two functional groups (CH3CHClSO2Cl, FSO2(CH2)nSO2F, n = 3, 4) and their application are listed in [3,9].
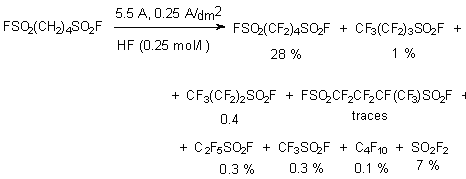
Electrochemical fluorination of alkanesulfonic acids passes through preliminary formation of fluoroanhydrides.
At that the length of carbon backbone chain influences the yield of base perfluorinated products
and when this length increases the yield decreases. Thus trifluoromethanesulfofluoride is obtained
with the 96% yield, C2F5SO2F - 61% and C8F17SO2F
-25% [10a].
Chloronhydrides of alkanesulfonic acids can be used as start substartes too [10b].
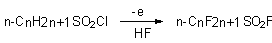

The main by-product of this reaction is perfluoroparaffin with the same number of carbon atoms. Along
with shortening of carbon backbone chain, leading to formation of perfluoroalkanesulfonic acids fluoroanhydrides
with lesser number of carbon atoms the sulfurylfluoride and sulfur hexafluoride are formed. Base
product yield varies from 5 to 40%. This explains the influence of functional groups on transformation
of carbon skeleton and the formation of partly fluorinated products at closing stages of fluorination.
If there are two SO2F groups in molecule, for example CH2(SO2F)2 [11-16], 1,2- and 1,3-propanedisulfonylfluorides, electrochemical fluorination in anhydrous fluoride hydrogen (current density 0.2 A/m2 , voltage 3.75-6 V) results in formation of base products with yields high enough. Perfluoroalkanesulfonic acids fluoroanhydride is obtained by implication, subjecting the appropriate acid to electrochemical fluorination in electrolyzer with electrodes made from nickel in anhydrous HF. Thus fluoroanhydride of difluoromethanesulfonic acid is obtained with the yield 75-82% (effectiveness of using electric energy is 33%). Fluoroanhydrides of 1,3-propane-, 1,3-butane- and 1,4-butanedisulfo-acids are obtained analogously to this [17,18]. It is determined that during the electrochemical fluorination process anode corrode 40 times faster than cathode, at that the corrosion is growing arcwise with the growth of anode tension. Because of this the development of convenient method of synthesis of dialkanesulfonic acids fluoroanhydrides is an important industrial task.
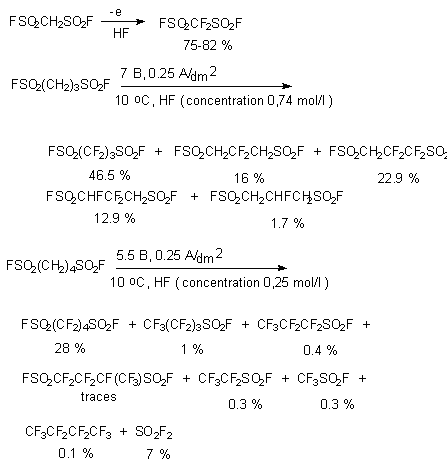
At that the character of alkyl row doesn't matter.
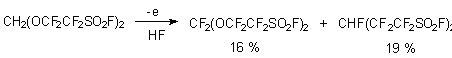
Nitrogen-containing functional group in linear part of alkanesulfonic acid doesn't influence in principle the passing of the electrochemical fluorination process [17-20].
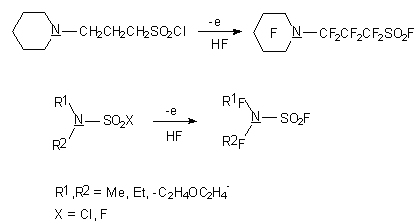
During the electrochemical fluorination of sultones the uncovering of cycle and fluoroanhydrides of sulfonic
and carboxylic acids formation take place. Thus 1,3-propanesultone at electrochemical fluorination
is turned into fluoroanhydride of 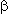 -fluorosulfonylbutanoic
acid [21].
-fluorosulfonylbutanoic
acid [21].
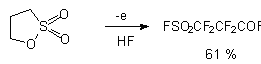
In the case of 2,5-dihydrothiophene-1,1-dioxide the uncovering of the cycle with the formation of perfluorobutansulfonic acid fluoroanhydride but with preserving of heterocyclic system takes place [22].
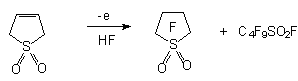
 ,
, -Difluoro-
-Difluoro- 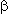 -sultones,
which are obtained from commercial available perfluoroolefines, during electrochemical fluorination
yield appropriate perfluoroalkanesulfonyl fluorides [23].
-sultones,
which are obtained from commercial available perfluoroolefines, during electrochemical fluorination
yield appropriate perfluoroalkanesulfonyl fluorides [23].
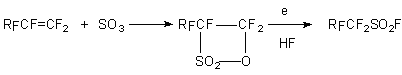
In the case of sultone on the basis of tetrafluoroethylene perfluoromethanesulfonyl fluoride, COF2,
CH4 and SO2F2 are obtained. This method is used for obtaining of
perfluoroethanesulfonyl fluoride and perfluorobutansulfonyl fluoride.
One of the methods of perfluoroalkanesulfonic acids obtaining extensively used in the chemistry of organofluorine compounds is the transformation of perfluoroalkyliodides. As the iodine movability in these compounds is not high enough, at first lithium salts are obtained, which react with sulfur dioxide, producing lithium salts of perfluoroalkansulfinic acid. The last mentioned oxidize in acetic acid at temperature of 100 oC by forming lithium salt of perfluoroalkanesulfonic acid. Usually such acids is obtained by reaction of lithium salt with concentrated sulphuric acid [24].
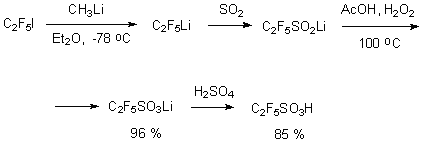
However this method is used only for lower acids, even perfluoropropyliodide doesn't produce the base product. Here the limiting stage is the obtaining of trifluoromethyl-lithium and pentafluoroethyl-lithium. The oxidization of perfluoroalkansulfinic acid salts can be conducted by hydrogen peroxide. However in this case the formation of perfluoroalkancarboxylic acid is registered with limited for one unit carbon backbone chain [25].
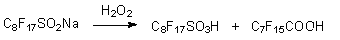
The use of perfluoralkyl iodides in the synthesis of sulfinic acids is inseparably linked with works of Chinese researchers on sulfinatehalogenation of halogen containing hydrocarbons. Thus they were the first to state, that perfluoralkyl iodides are able to react with Na2S2O4 in the presence of Lewis' basis (NaHCO3, Na2CO3) in aprotic solvents (DMF, N-methylpyrrolidone, HMTPA) at 40-120 oC and at the reaction period 1-10 hours, the result of this was the synthesis of sulfinic acid derivatives. The review of perfluoroalkanesulfinate chemistry, including their obtaining , characteristics, reactions, and application is given in the works [33-35].
Using the cheap reducer (for example Na2S2O4 ) one can obtain salts of perfluoroalkansulfinic acids from perfluoroalkyl halides (RFX, where X=Br,I , RFCCl3) in mild conditions. The fact, that this method is extensively used for carrying out the reactions of mentioned salts with olefins, dienes, acetylene derivatives and aromatic compounds is more important. Along with that the present system is effective mainly for polyfluorinated alkyl halides [27]. Bromine and iodine situated in perfluorinated carbon backbone chain are easily replaced and base product is formed with high yield. In future such works were carried out by other researchers, who confirmed the effectiveness of this methodology [36-50].
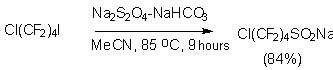
Later it was shown that another sulfur compounds, possessing nucleofilic properties, are able to react with perfluoralkyl iodides forming salts of perfluoroalkansulfinic acid (table 1) [42,51-54]. Obviously, these reactions pass according to one-electron transfer mechanism (SET), the catching of perfluoralkyl radical by olefins and nitrosocompounds is the confirmation of this fact [26].
The process of sulfonylization by Na2S2O4 appeared to be important, because it allows to obtain the corresponding perfluoroalkanesulfonic acids, which there are important intermediate components for surfactant synthesis, omitting the electrochemical fluorination stage and using available perfluoroalkyliodides [28,42,55,56]. For example, X(CF2)2nO(CF2)2SO3Na (X = Br, I, n =1,2), I(CF2)2O(CF2)COONa, I(CF2)nO(CF2)nSO3Na can be obtained from corresponding perfluoroalkyl halides Cl(CF2)nI (n = 2,4,6), H(CF2)8X (X = Br, I); Br(CF2)4Cl and I(CF2)2O(CF2)2I in the presence of phase-transfer catalyst in polyethylene glycols 200 and -600 or in acetonitrile, ethanol, diglyme.
Also, this reaction can be carried out with perfluoroalkylbromides, for example CF3Br, which produces sodium salt of trifluoromethansulfinic acid with 90% yield. 1,1,1-Trichloroperfluoroalkanes appeared adequate for these purposes (product yields 90% and more) [37-31, 57,58].
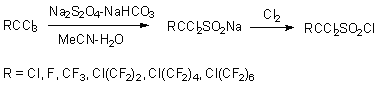
Triethylamine was used as a base [30].
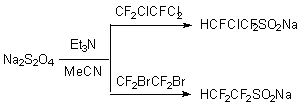
The reactions of  ,
,
 -diiodoperfluoroalkanes [41] and
-diiodoperfluoroalkanes [41] and  ,
,
 -dibromoperfluoroalkanes with equivalent amount of Na2S2O4in
the media MeCN-H2O in the presence of sodium bicarbonate result in mixtures
of sodium salts of perfluoroalkane-
-dibromoperfluoroalkanes with equivalent amount of Na2S2O4in
the media MeCN-H2O in the presence of sodium bicarbonate result in mixtures
of sodium salts of perfluoroalkane- ,
,
 -sulfinates (yields 66-90%)(see table 2).
-sulfinates (yields 66-90%)(see table 2).
I(CF2)nSO2Na and NaSO2(CF2)nSO2Na at chlorine action at 0oC produce appropriate sulfochlorides I(CF2)nSO2Cl and ClSO2(CF2)nSO2Cl [n = 3,4,6].
Table 1. Synthesis of perfluoroalkansulfinic acid sodium salts

Table 2. Sulfinilizing and subsequent chlorination of
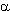 ,
, -diiodoperfluoroalkanes I(CF2)nI [41,47].
-diiodoperfluoroalkanes I(CF2)nI [41,47].
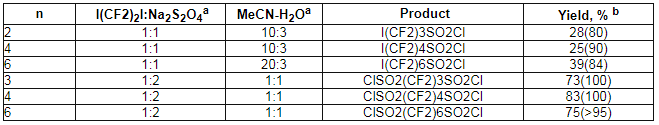
amole ratio
bin parentheses the yields from NMR 19F information
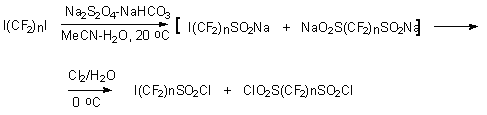
The mechanism of this process can be presented as follows:
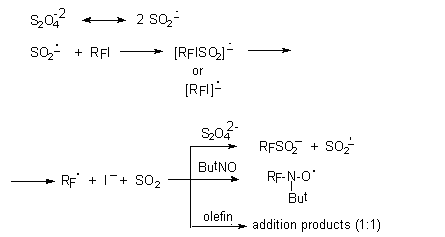
The sulfinatedehalogenation reaction had opened a new way to the synthesis of perfluoroalkane-sulfinic
and -sulfonic acids and their derivatives. It is interesting, because perfluoroalkyl
halides is transformed directly into perfluoroalkylsulfinate. In this case there
is no need of intermediate section – the synthesis of organometallic derivative.
This process is surely interesting for extensive application in the technology [27-30,36-39,58].
to be continued
Fluorine Notes, 2003, 27, 1-2
